EHF inhibits pancreatic cancer stemness by blocking the activity of Wnt/β-catenin pathway
-
摘要:
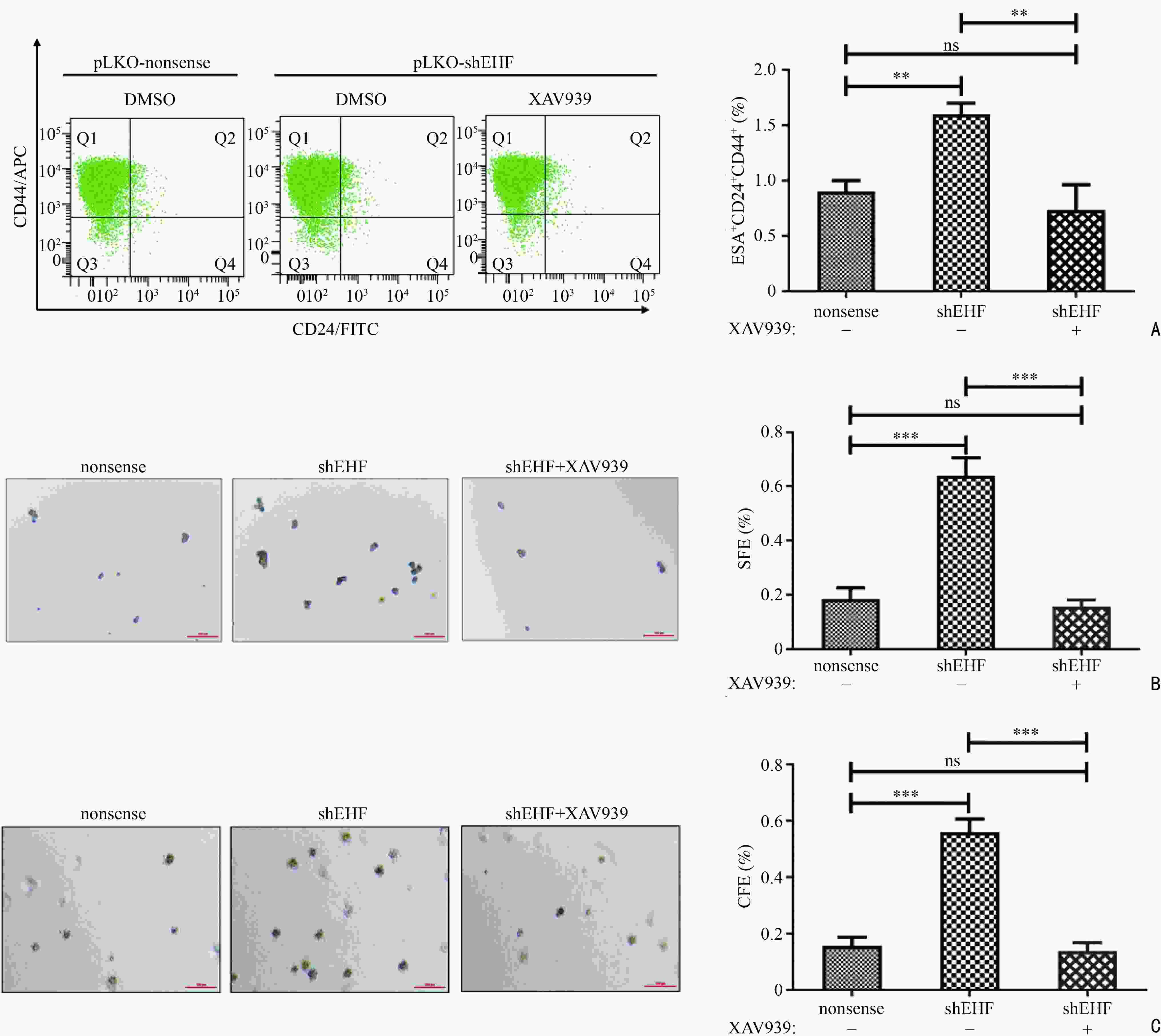
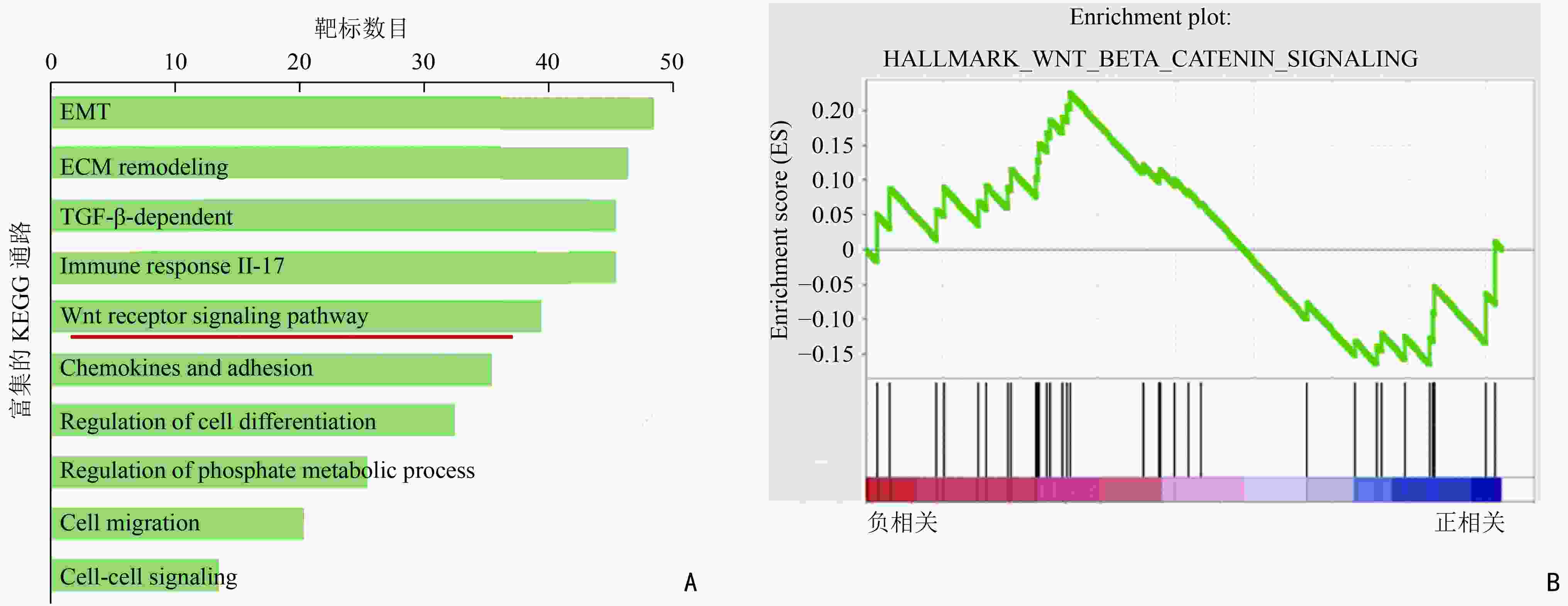
目的 探索E26转化特异性同源因子(E26 transformation-specific homologous factor,EHF)调控胰腺癌细胞干性的机制。 方法 通过生信分析寻找介导EHF调控胰腺癌干性的信号通路。利用Western blot及RT-qPCR技术验证胰腺癌细胞中EHF表达与目标干性通路活性的相关性。利用ChIP及双荧光素酶实验验证EHF对靶基因的调控作用。阻断通路活性后,利用功能学实验分析目标通路在EHF调控胰腺癌细胞干性过程中的作用。 结果 生信分析发现Wnt/β-catenin通路参与EHF对胰腺癌干性的调控。构建EHF稳定过表达及稳定降表达的胰腺癌细胞系,通过Western blot及RT-qPCR技术证实胰腺癌细胞EHF的表达与Wnt/β-catenin通路的活性呈负相关,差异均具有统计学意义(均P<0.01)。EHF可通过结合β-catenin的启动子区直接抑制β-catenin的转录。通过流式细胞术、悬浮成球及软琼脂克隆形成实验证明,在利用XAV939阻断Wnt/β-catenin通路的活性后,由EHF敲低所引起的胰腺癌细胞干性上调可被有效抑制,差异均具有统计学差异(均P<0.01)。 结论 EHF通过抑制Wnt/β-catenin通路活性下调胰腺癌细胞的干性,靶向Wnt/β-catenin通路的小分子抑制剂有潜力成为治疗胰腺癌的药物。 -
关键词:
- 胰腺癌 /
- 干性 /
- EHF /
- Wnt/β-catenin通路 /
- XAV939
Abstract:Objective To explore the mechanism underlying E26 transformation-specific homologous factor (EHF) in regulating the stemness of pancreatic cancer cells. Methods Bioinformatic analyses were conducted to identify the downstream pathway regulating the stemness of pancreatic cancer cells through EHF. Western blot and RT-qPCR were used to verify the correlation between EHF expression and the activity of the selected stemness pathway in pancreatic cancer. ChIP and double luciferase reporter gene experiments were used to verify the function of EHF in regulating the target genes. After treatment with pathway inhibitors, functional experiments were performed to determine the importance of the selected pathway in the EHF-mediated regulation of pancreatic cancer stemness. Results Bioinformatic analyses revealed that the Wnt/β-catenin pathway mediates the stemness of pancreatic cancer cells through EHF regulation. Pancreatic cells with stably overexpressed or downregulated EHF were constructed. Further Western bolt and RT-qPCR verified that EHF expression was negatively correlated to the activity of the Wnt/β-catenin pathway in pancreatic cancer cells (P<0.01). The direct binding of EHF to the promoter region of β-catenin could ultimately inhibitits transcription. After the treatment with XAV939, an inhibitor of the Wnt/β-catenin pathway, functional experiments including flow cytometry, suspension cell spheroidization, and soft agar clone formation indicated that the upregulation of stemness characteristics caused by EHF knockdown could be effectively inhibited (P<0.01) in pancreatic cancer. Conclusions EHF inhibits the stemness of pancreatic cancer cells by blocking Wnt/β-catenin pathway. Small molecule inhibitors targeting Wnt/β-catenin pathway have the potential to treat pancreatic cancer in future. -
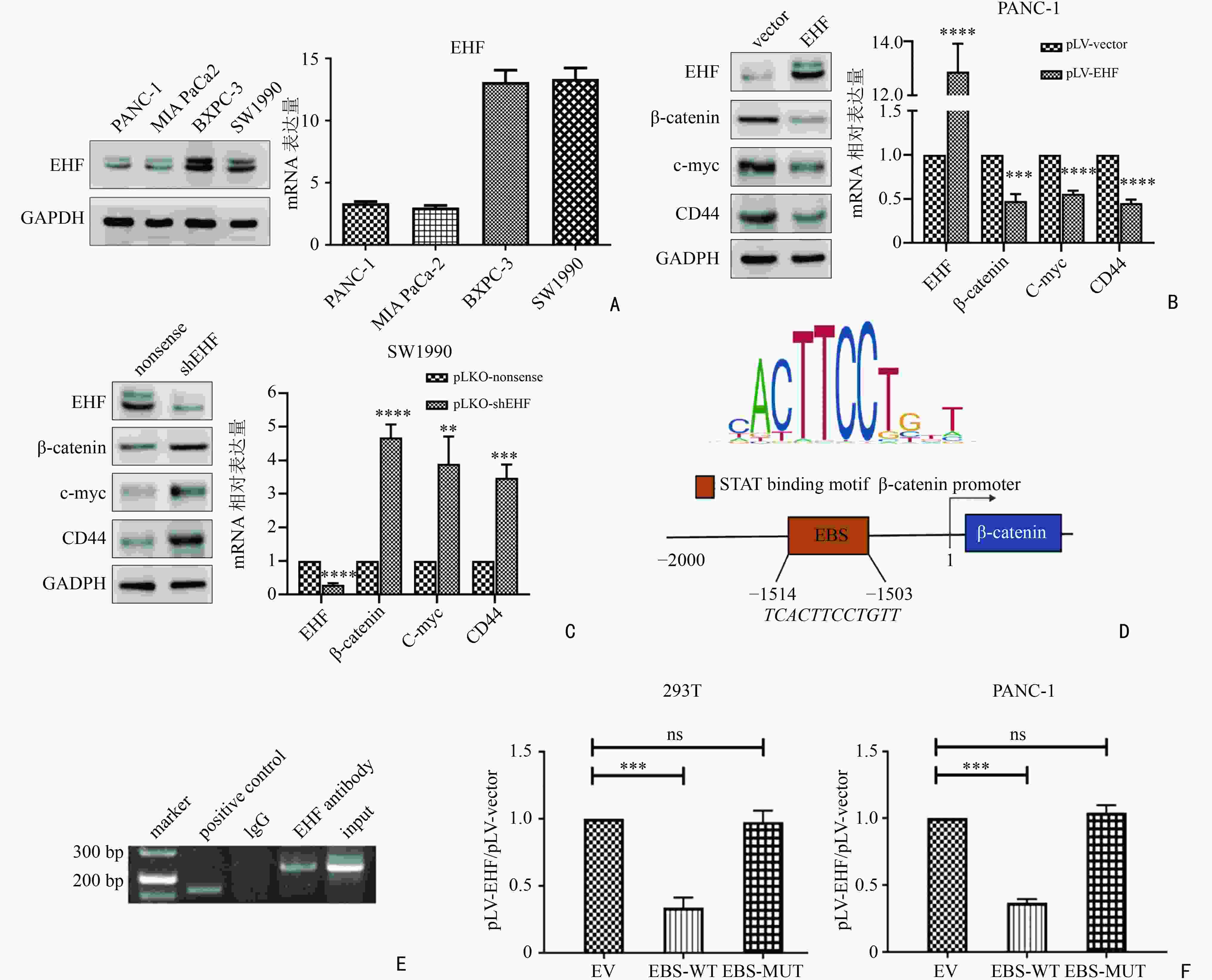
图 2 EHF通过抑制β-catenin的转录影响Wnt/β-catenin通路的活性
A:不同胰腺癌细胞系中EHF的蛋白水平存在差异;B:EHF表达上调后,β-catenin、c-myc及CD44的表达均出现下降;C:EHF敲低后,β-catenin、c-myc及CD44的表达均出现上升;D:EHF可能与β-catenin启动子区结合的位置;E:EHF可结合在β-catenin的启动子区;F:EHF可直接抑制β-catenin的转录。EBS-WT:EHF在启动子区的结合序列野生型;EBS-MUT:EHF在启动子区的结合序列突变型:ns:差异无统计学意义;**:P<0.01;***:P<0.001;****:P<0.000 1
表 1 RT-qPCR的引物序列
基因名称 引物序列 EHF F: 5' -CTCGAGCTAGACCGTGTCCACCT-3' R: 5' - GTAGGCCGCTATGGACTGTGCAAT-3' β-actin F: 5' - AGGCCAACCGCGAGAAGATGACC-3' R: 5' - GAAGTCCAGGGCGACGTAGCACC-3' β-catenin F: 5' -CGCTAAAGTTCTCTGGATCCACCT-3' R: 5' - GTAGGCCGCTATGGACTGTGCAAT-3' c-myc F: 5' -CCTTCTCTAACCTCTCCTGGATCCA-3' R: 5' - AGAGGTCTCTAATGCGTGAAGTGC-3' CD44 F: 5' -CGCTGTCATCCTCATTCACACTTGC-3' R: 5' - AGCTGCACACTCCACAGGAAGAAT-3' -
[1] Liu J, Jiang W, Zhao K, et al. Tumoral EHF predicts the efficacy of anti-PD1 therapy in pancreatic ductal adenocarcinoma[J]. J Exp Med, 2019, 216(3):656-673. doi: 10.1084/jem.20180749 [2] Zhao T, Jiang W, Wang X, et al. ESE3 inhibits pancreatic cancer metastasis by upregulating E-Cadherin[J]. Cancer Res, 2017, 77(4):874-885. doi: 10.1158/0008-5472.CAN-16-2170 [3] Albino D, Longoni N, Curti L, et al. ESE3/EHF controls epithelial cell differentiation and its loss leads to prostate tumors with mesenchymal and stem-like features[J]. Cancer Res, 2012, 72(11):2889-2900. doi: 10.1158/0008-5472.CAN-12-0212 [4] Albino D, Civenni G, Rossi S, et al. The ETS factor ESE3/EHF represses IL-6 preventing STAT3 activation and expansion of the prostate cancer stem-like compartment[J]. Oncotarget, 2016, 7(47):76756-76768. doi: 10.18632/oncotarget.12525 [5] Zhou T, Liu J, Xie Y, et al. ESE3/EHF, a promising target of rosiglitazone, suppresses pancreatic cancer stemness by downregulating CXCR4[J]. Gut, 2022, 71(2):357-371. doi: 10.1136/gutjnl-2020-321952 [6] Gupta VK, Sharma NS, Durden B, et al. Hypoxia-driven oncometabolite L-2HG maintains stemness-differentiation balance and facilitates immune evasion in pancreatic cancer[J]. Cancer Res, 2021, 81(15):4001-4013. doi: 10.1158/0008-5472.CAN-20-2562 [7] Pan G, Liu Y, Shang L, et al. EMT-associated microRNAs and their roles in cancer stemness and drug resistance[J]. Cancer Commun (Lond), 2021, 41(3):199-217. doi: 10.1002/cac2.12138 [8] Romano S, Tufano M, D'Arrigo P, et al. Cell stemness, epithelial-to-mesenchymal transition, and immunoevasion: intertwined aspects in cancer metastasis[J]. Semin Cancer Biol, 2020, 60:181-190. doi: 10.1016/j.semcancer.2019.08.015 [9] Gene Ontology Consortium. Gene ontology consortium: going forward[J]. Nucleic Acids Res, 2015, 43:D1049-1056. doi: 10.1093/nar/gku1179 [10] Eraso-Pichot A, Brasó-Vives M, Golbano A, et al. GSEA of mouse and human mitochondriomes reveals fatty acid oxidation in astrocytes[J]. Glia, 2018, 66(8):1724-1735. doi: 10.1002/glia.23330 [11] Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer[J]. Oncogene, 2017, 36(11):1461-1473. doi: 10.1038/onc.2016.304 [12] Clevers H, Nusse R. Wnt/β-catenin signaling and disease[J]. Cell, 2012, 149(6):1192-1205. doi: 10.1016/j.cell.2012.05.012 [13] Jang J, Jung Y, Chae S, et al. XAV939, a Wnt/β-catenin pathway modulator, has inhibitory effects on LPS-induced inflammatory response[J]. Immunopharmacol Immunotoxicol, 2019, 41(3):394-402. doi: 10.1080/08923973.2018.1536984 [14] He W, Wu J, Shi J, et al. IL22RA1/STAT3 signaling promotes stemness and tumorigenicity in pancreatic cancer[J]. Cancer Res, 2018, 78(12):3293-3305. doi: 10.1158/0008-5472.CAN-17-3131 [15] Buckley AM, Lynam-Lennon N, O'Neill H, et al. Targeting hallmarks of cancer to enhance radiosensitivity in gastrointestinal cancers[J]. Nat Rev Gastroenterol Hepatol, 2020, 17(5):298-313. doi: 10.1038/s41575-019-0247-2 [16] Collins M, Soularue E, Marthey L, et al. Management of patients with immune checkpoint inhibitor-induced enterocolitis: a systematic review[J]. Clin Gastroenterol Hepatol, 2020, 18(6):1393-1403. doi: 10.1016/j.cgh.2020.01.033 [17] Masiak-Segit W, Rawicz-Pruszyński K, Skórzewska M, et al. Surgical treatment of pancreatic cancer[J]. Pol Przegl Chir, 2018, 90(2):45-53. doi: 10.5604/01.3001.0011.7493 [18] Okusaka T, Furuse J. Recent advances in chemotherapy for pancreatic cancer: evidence from Japan and recommendations in guidelines[J]. J Gastroenterol, 2020, 55(4):369-382. doi: 10.1007/s00535-020-01666-y [19] Crippa S, Cirocchi R, Weiss MJ, et al. A systematic review of surgical resection of liver-only synchronous metastases from pancreatic cancer in the era of multiagent chemotherapy[J]. Updates Surg, 2020, 72(1):39-45. doi: 10.1007/s13304-020-00710-z [20] Knutson S, Raja E, Bomgarden R, et al. Development and evaluation of a fluorescent antibody-drug conjugate for molecular imaging and targeted therapy of pancreatic cancer[J]. PloS One, 2016, 11(6):e0157762. doi: 10.1371/journal.pone.0157762 [21] Xie C, Duffy A, Brar G, et al. Immune checkpoint blockade in combination with stereotactic body radiotherapy in patients with metastatic pancreatic ductal adenocarcinoma[J]. Clin Cancer Res, 2020, 26(10):2318-2326. doi: 10.1158/1078-0432.CCR-19-3624 [22] Lin QJ, Yang F, Jin C, et al. Current status and progress of pancreatic cancer in China[J]. World J Gastroenterol, 2015, 21(26):7988-8003. doi: 10.3748/wjg.v21.i26.7988 [23] Ren B, Cui M, Yang G, et al. Tumor microenvironment participates in metastasis of pancreatic cancer[J]. Mol Cancer, 2018, 17(1):108. doi: 10.1186/s12943-018-0858-1 [24] Xie SL, Fan S, Zhang SY, et al. SOX8 regulates cancer stem-like properties and cisplatin-induced EMT in tongue squamous cell carcinoma by acting on the Wnt/β-catenin pathway[J]. Int J Cancer, 2018, 142(6):1252-1265. doi: 10.1002/ijc.31134 [25] Quayle LA, Ottewell PD, Holen I. Chemotherapy resistance and stemness in mitotically quiescent human breast cancer cells identified by fluorescent dye retention[J]. Clin Exp Metastasis, 2018, 35(8):831-846. doi: 10.1007/s10585-018-9946-2 [26] Yeh HW, Hsu EC, Lee SS, et al. PSPC1 mediates TGF-β1 autocrine signalling and Smad2/3 target switching to promote EMT, stemness and metastasis[J]. Nat Cell Biol, 2018, 20(4):479-491. doi: 10.1038/s41556-018-0062-y [27] Liu YL, Stadler ZK. The future of parallel tumor and germline genetic testing: is there a role for all patients with cancer[J]. J Natl Compr Canc Netw, 2021, 19(7):871-878. doi: 10.6004/jnccn.2021.7044 [28] Ignatiadis M, Sledge GW, Jeffrey SS. Liquid biopsy enters the clinic - implementation issues and future challenges[J]. Nat Rev Clin Oncol, 2021, 18(5):297-312. doi: 10.1038/s41571-020-00457-x -



 下载:
下载: