Clinical significance of histological classification in prognostic evaluation ofappendiceal mucinous tumors treated with CRS combined with HIPEC
-
摘要:
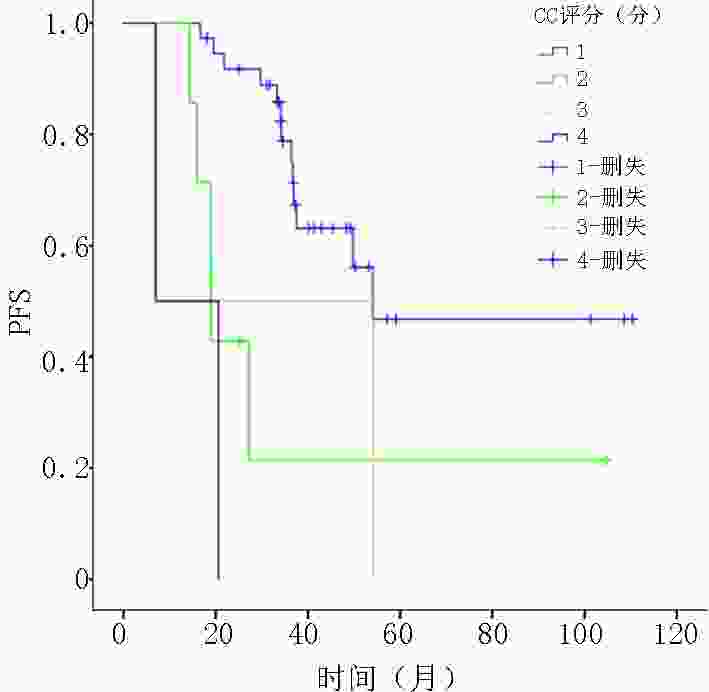
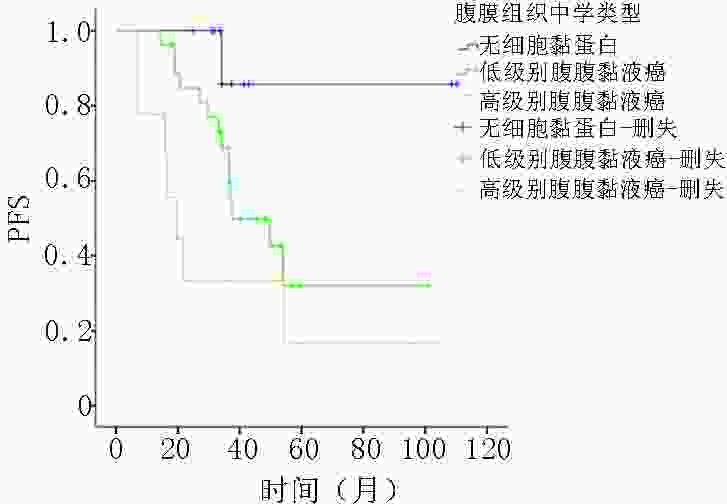
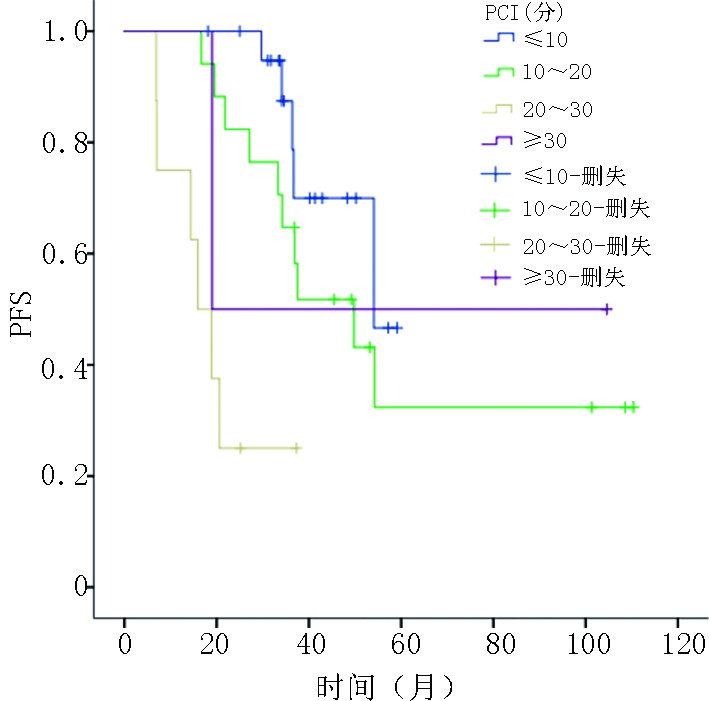
目的 阑尾黏液性肿瘤较为罕见,可发生腹膜转移形成腹膜假黏液瘤。目前,临床上多应用肿瘤细胞减灭术(cytoreductive surgery,CRS)和腹腔热灌注化疗(hyperthermic introperitoneal chemotherapy,HIPEC)进行治疗。其组织学类型、腹膜癌指数(peritoneal carcinomatosis index,PCI)评分及细胞减灭程度(completeness of cytoreduction,CC)评分等因素和预后的相关性尚为明确。将规范的组织学分类作为预测因子应用至临床,探讨不同组织学分型、PCI评分和CC评分等因素对伴有腹膜转移的阑尾黏液性肿瘤的预后影响。 方法 回顾行分析2009年3月至2019年1月重庆大学附属肿瘤医院就诊的经CRS联合HIPEC治疗后的阑尾黏液性肿瘤的转归。按照2019年第5版世界卫生组织(WHO)对消化道肿瘤推荐的分类标准和国际腹膜表面肿瘤组的规范化组织学分型,采用Cox比例风险模型,通过单变量和多变量分析明确组织学分型、PCI评分、CC评分对患者无进展生存期(progress free survival, PFS)的影响。 结果 共48名患者接受了CRS+HIPEC的治疗。经单因素Cox回归分析,PCI 评分 、CC评分、原发组织学类型和腹膜组织学类型均对PFS存在影响,差异具有统计学意义(P<0.05)。表现为与PCI评分≤10分相比,20~30的危险比为10.38;CC评分与0分相比,1分和3分的危险比分别为4.26和14.74;原发组织学类型中与低级别黏液性肿瘤相比,印戒细胞癌的危险比为9.81;腹膜组织学类型中,与无细胞黏蛋白相比,高级别腹膜黏液癌的危险比为14.35。经多因素Cox回归分析,仅原发组织学类型对PFS存在影响,差异具有统计学意义(P<0.05),原发组织学类型中与低级别黏液性肿瘤相比,印戒细胞癌的危险比为110.79。 结论 对于经过CRS+HIPEC治疗阑尾黏液性肿瘤及其引起的腹膜假黏液瘤,规范化地进行原发病灶和腹膜病灶组织学分型对患者预后评估具有重要意义。腹膜病灶组织学恶性程度与原发病灶组织学恶性程度相比呈正相关。原发病灶和腹膜灶恶性度越高,患者预后越差。相对于腹膜病灶组织学分型,原发病灶组织学分型与患者预后更为密切,是更好的预测指标。同时,患者预后与CRS的CC评分和PCI评分有关,PCI评分和CC评分越高,患者预后越差。因此,在CRS+HIPEC治疗中,应对原发病灶和腹膜病灶进行组织学分型,尽可能减瘤彻底,规范化地HIPEC治疗使患者获益。 Abstract:Objective Appendiceal mucinous tumors are rare, which can cause peritoneal metastasis and form pseudomyxoma peritonei (PMP). Currently, cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) are usually adopted for treating appendiceal mucinous tumors. However, correlations between histological type, peritoneal carcinomatosis index (PCI) score, and completeness of cytoreduction (CC) score and prognosis remain unclear. Thus, this study aimed to investigate the effect of histological classification, PCI score, and CC score on the prognosis of patients with appendiceal mucinous tumors with peritoneal metastasis. Methods Data on outcomes of appendiceal mucinous tumors treated with CRS combined with HIPEC at Chongqing University Cancer Hospital from March 2009 to January 2019 were reviewed. According to the digestive system classification criteria (5th edition, 2019) recommended by the World Health Organization (WHO) and the Peritoneal Surface Oncology Group International (PSOGI), a Cox proportional hazard model was used to determine the effect of tissue classification, PCI score, and CC score on the progression-free survival (PFS) of the patients. Results In total, 48 patients were treated with CRS combined with HIPEC. In the univariate Cox regression analysis, PCI score, CC score, primary histological type, and peritoneal histological type had statistical effects on PFS ( P<0.05). Compared with PCI score≤ 10, the risk ratio of 20-30 scores was 10.38. Compared with 0, the risk ratios of CC score 1 and 3 were 4.26 and 14.74, respectively. The risk ratio of signet-ring cell carcinoma in patients with the primary histological type was 9.81 compared with that of low-grade mucinous tumors, while the risk ratio of high-grade mucinous adenocarcinoma was 14.35 compared with that of acellular mucin tumors. In the multivariate Cox regression analysis, only primary histological type had a significant effect on PFS ( P<0.05). The risk ratio of signet-ring cell carcinoma was 110.79 in patients with the primary histological type compared with that of low-grade mucinous tumors. Conclusions Standardizing the histological classification of primary and peritoneal lesions for the prognostic evaluation of patients with appendiceal mucinous tumors and peritoneal pseudomyxoma treated with CRS combined with HIPEC is of great importance. The histological malignant degree of peritoneal lesions was positively correlated with that of primary lesions, and the higher malignancy of primary and peritoneal lesions was attributed to a worse patient prognosis. Compared with the histological type of peritoneal lesions, that of primary lesions was more closely related to patient prognosis and was therefore a better predictor. Additionally, patient prognosis was related to CC and PCI scores of patients treated with CRS, and higher PCI and CC scores were attributed to a worse patient prognosis. Therefore, to treat patients with CRS combined with HIPEC, we should standardize the histological classification of primary and peritoneal lesions, reduce the tumor completely, and normatively administer HIPEC for the patients’ full benefit. -
表 1 基线及组织学数据
变量 中位数
(范围或n%)CRS联合HIPEC治疗总数 48(100) 年龄 (岁) 45(26~72) 性别 男 26(54.2) 女 22(45.8) 阑尾原发灶组织学分型 低级别黏液性肿瘤 23(47.9) 高级别黏液性肿瘤
(不含印戒细胞成分的浸润性黏液癌)14(29.2) 印戒细胞癌 11(22.9) 腹膜组织学分型 良性(无黏液及肿瘤细胞) 1(2.1) 无细胞黏蛋白 11(22.9) 低级别腹膜黏液性肿瘤 27(56.3) 高级别腹膜黏液癌 9(18.8) PCI评分(分) ≤10 21(43.8) 11~20 17(35.4) 21~30 8(16.7) ≥30 2(4.2) CC评分(分) 0 37(77.1) 1 7(14.6) 2 2(4.2) 3 2(4.2) 表 2 48例原发病灶及腹膜病灶组织学分析
阑尾原发灶组织学 腹膜转移灶组织学 良性(n=1) 无细胞黏液蛋白(n=11) 低级别腹膜黏液性肿瘤(n=27) 高级别腹膜黏液癌(n=9) 低级别黏液性肿瘤(n=23) 1(4.3) 7(30.4) 15(65.2) 0(0) 高级别黏液性肿瘤(n=14) 0(0) 4(28.6) 7(50.0) 3(21.4) 印戒细胞癌(n=11) 0(0) 0(0) 5(45.5) 6(54.5) 表 3 PFS的单变量和多变量分析
原发灶组织学 例数 进展n(%) mPFS(月)(95% CI) 单因素分析 多因素分析 危险比(95%CI) P 危险比(95%CI) P 总例数(n=48) 22(45.8) 49.8(29.4,70.2) PCI评分(分) 0.002 0.108 ≤10 21 5(23.8) 54.1(−) − − − − 10~20 17 10(58.8) 49.8(28.1,71.5) 2.07(0.70,6.09) 0.188 0.98(0.19,4.98) 0.977 20~30分 8 6(75.0) 15.9(9.7,22.1) 10.38(3.01,35.72) 0.000 3.12(0.24,40.27) 0.383 ≥30 2 1(50.0) 19.0(−) 1.73(0.20,15.25) 0.620 0.13(0.01,4.31) 0.255 CC评分 0.002 0.340 0 37 13(35.1) 54.1(−) − − − − 1 7 5(71.4) 19.0(18.7,19.3) 4.26(1.47,12.32) 0.008 0.10(0.01,1.22) 0.071 2 2 2(100) 6.9(−) 3.04(0.67,13.88) 0.151 1.10(0.13,9.35) 0.928 3 2 2(100.0) 7.0(−) 14.74(2.89,75.19) 0.001 0.12(0.01,3.19) 0.202 阑尾原发组织学类型 0.000 0.006 低级别黏液性肿瘤 23 6(26.1) − − − − − 高级别黏液性肿瘤 14 7(50.0) 49.8(33.3,66.3) 2.11(0.71,6.31) 0.179 2.03(0.40,10.22) 0.392 印戒细胞癌 11 9(81.8) 18.9(15.6,22.2) 9.81(3.33,28.83) 0.000 110.79(5.68,2 161.07) 0.002 腹膜组织学类型 0.023 0.262 无细胞黏蛋白 11 1(9.1) − − − − − 低级别腹膜黏液性肿瘤 27 14(51.9) 37.5(21.7,53.3) 5.88(0.77,44.78) 0.087 6.26(0.69,56.49) 0.102 高级别腹膜黏液癌 9 7(77.8) 19.5(11.3,27.7) 14.35(1.75,117.75) 0.013 5.93(0.44,80.26) 0.181 -
[1] Ploenes T, Börner N, Kirkpatrick CJ, et al. Neuroendocrine tumour, mucinous adenocarcinoma and signet-ring cell carcinoma of the appendix: three cases and review of literature[J]. Indian J Surg, 2013, 75(Suppl 1):299-302. [2] Shaib WL, Goodman M, Chen Z, et al. Incidence and survival of appendiceal mucinous neoplasms: a seer analysis[J]. Am J Clin Oncol, 2017, 40(6):569-573. doi: 10.1097/COC.0000000000000210 [3] Chua TC, Moran BJ, Sugarbaker PH, et al. Early-and long-term outcome data of patients with pseudomyxoma peritonei from appendicealorigin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy[J]. J Clin Oncol, 2012, 30(20):2449-2456. doi: 10.1200/JCO.2011.39.7166 [4] Austin F, Mavanur A, Sathaiah M, et al. Aggressive management of peritoneal carcinomatosis from mucinous appendiceal neoplasms[J]. Ann Surg Oncol, 2012, 19(5):1386-1393. doi: 10.1245/s10434-012-2241-6 [5] Nagtegaal ID, Klimstra DS, Wshington MK. The 2019 WHO classification of tumours of the digestive system[M]. IARC Press, 2019:136-156. [6] Carr NJ, Bibeau F, Bradley RF, et al. The histopathological classification, diagnosis and different diagnosis of mucinousappendiceal neoplasms, appendiceal adenocarcinomas and pseudomyxoma peritonei[J]. Histopathology, 2017, 71(6):847-858. doi: 10.1111/his.13324 [7] Baumgartner JM, Tobin L, Heavey S, et al. Predictors of progression in high-grade appendiceal or colorectal peritoneal carcinomatosis aftercytoreductive surgery and hyperthermi intraperitoneal chemotherapy[J]. Ann Surg Oncol, 2015, 22(5):1716-1721. doi: 10.1245/s10434-014-3985-y [8] Portilla AG, Shigeki K, Dario B, et al. The intraoperative staging systems in the management of peritoneal surface malignancy[J]. J Surg Oncol, 2008, 98(4):228-231. doi: 10.1002/jso.21068 [9] Woeste MR, Philips P, Egger ME, et al. Optimal perfusion chemotherapy: a prospective comparison of mitomycin C and oxaliplatin for hyperthermic intraperitoneal chemotherapy in metastatic colon cancer[J]. J Surg Oncol, 2020, 121(8):1298-1305. doi: 10.1002/jso.25920 [10] Yoshino T, Arnold D, Taniguchi H, et al. Pan-asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS[J]. Ann Oncol, 2018, 29(1):44-70. doi: 10.1093/annonc/mdx738 [11] Carr NJ, Cecil TD, Mohamed F, et al. A consensus for classification and pathologic reporting of pseudomyxoma peritonei and associated appendiceal neoplasia: the results of the peritoneal surface oncology group international (PSOGI) modified delphi process[J]. Am J Surg Pathol, 2016, 40(1):14-26. doi: 10.1097/PAS.0000000000000535 [12] Gündoğar Ö, Kımıloğlu E, Komut N, et al. Evaluation of appendiceal mucinous neoplasms with a new classification system and literature review[J]. Turk J Gastroenterol, 2018, 29(5):533-542. [13] XJ Yang, CQ Huang, Tao Suo, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase Ⅲ randomized clinical trial[J]. Ann Surg Oncol, 2011, 18(6):1575-1581. doi: 10.1245/s10434-011-1631-5 [14] Villeneuve L, Isaac S, Glehen O, et al. The RENAPE network: towards a new healthcare organi-zation for the treatment of rare tumors of the peritoneum. Description of the network and role of the pathologists[J]. Ann Pathol, 2014, 34(1):4-8. doi: 10.1016/j.annpat.2014.01.008 [15] Enblad M, Birgisson H, Wanders A, et al. Importance of absent neoplastic epithelium in patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy[J]. Ann Surg Oncol, 2016, 23(4):1149-1156. doi: 10.1245/s10434-015-4989-y [16] Huang Y, Alzahrani NA, Chua TC, et al. Histological subtype remains a significant prognostic factor for survival Outcomes in patients with appendiceal mucinous neoplasm with peritoneal dissemination[J]. Dis Colon Rectum, 2017, 60(4):360-367. doi: 10.1097/DCR.0000000000000719 [17] Winer J, Zenati M, Ramalingam L, et al. Impact of aggressive histology and location of primary tumor on the efficacy of surgical therapy for peritoneal carcinomatosis of colorectal origin[J]. Ann Surg Oncol, 2014, 21(5):1456-1462. doi: 10.1245/s10434-013-3328-4 [18] Wagner PL, Austin F, Sathaiah M, et al. Significance of serum tumor markers levels in peritoneal carcinomatosis of appendiceal origin[J]. Ann Surg Oncol, 2013, 20(2):506-514. doi: 10.1245/s10434-012-2627-5 -




 下载:
下载: