Effect of Keap-1/Nrf2/HO-1 pathway on inhibition of cisplatin induced myocardial injury in rats by trimetazidine
-
摘要:
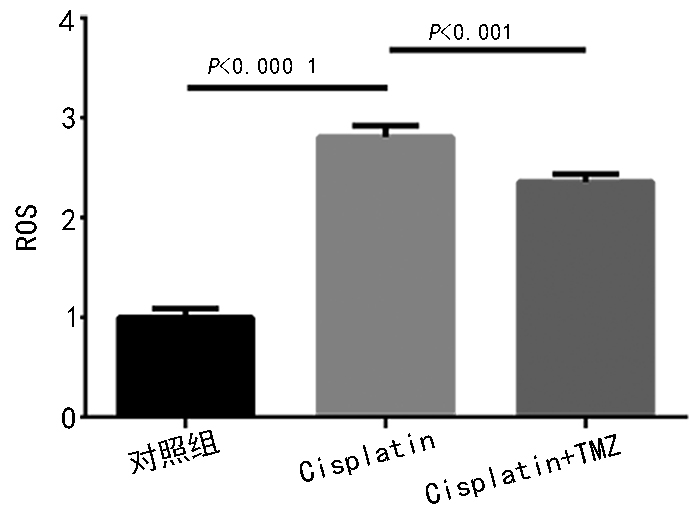
目的 研究曲美他嗪(trimetazidine,TMZ)减轻顺铂(cisplatin)诱导的大鼠心肌细胞损伤,并通过抑制Keap-1调控Nrf2/HO-1起到心脏保护作用。 方法 分离大鼠心肌细胞,分成三组,对照组、顺铂处理组(200 μM)和顺铂(200 μM)+TMZ(200 μM)处理组。检查细胞活力和ROS的含量,Western blot及Real-time PCR方法检测Nrf2及其上游Keap-1和下游HO-1的表达。 结果 TMZ明显减轻顺铂对心肌细胞活力的抑制(P<0.01)。TMZ预处理均能有效地抑制活性氧物质ROS(P<0.01)的水平。Western blot结果显示,TMZ预处理可明显降低Nrf2的表达(P<0.01),抑制Keap-1的蛋白表达(P<0.01),增强抗氧化酶类靶蛋白如血红素氧合酶 1(hemo oxygenase 1,HO-1)的蛋白表达水平(P<0.01)。Real-time PCR结果显示,与对照组相比,顺铂处理的Nrf2表达显著增加(P<0.0001),TMZ预处理明显促进了Nrf2的核易位(P<0.01);顺铂显著促进Keap-1(P<0.0001)和HO-1的表达(P<0.0001),TMZ进行预处理可显著抑制Keap-1的表达(P<0.0001),显著增强HO-1的表达水平(P<0.001)。 结论 TMZ抑制了顺铂引起的心脏损伤,部分依赖于Keap-1/Nrf2/HO-1信号通路的抗氧化防御作用,显示出TMZ具有心脏保护作用,可作为抗氧化剂治疗化疗药物引起的心脏损伤。 Abstract:Objective To study the effect of trimetazidine (TMZ) in reducing rat myocardial injury induced by cisplatin and the cardioprotective effect of Nrf2/HO-1 by inhibiting Keap-1. Methods Rat myocardial cells were isolated and divided into three groups: control group, cisplatin treated group (200 μM), and cisplatin +TMZ (200 μM) treated group. Cell viability and production of reactive oxygen species (ROS) were examined. Western blot and Real-time PCR were used to detect the expression of Nrf2, its upstream Keap-1, and downstream HO-1. Results TMZ reduced the inhibitory effect of cisplatin on cardiomyocyte activity (P<0.01). TMZ pretreatment could effectively inhibit ROS production (P<0.01). Western blot results showed that TMZ pretreatment increased the expression of Nrf2 (P<0.01), inhibited the expression of Keap-1 (P<0.01), and enhanced HO-1 expression level (P<0.01). Compared with the control group, Real-time PCR revealed that the expression of Nrf2 was significantly increased by cisplatin treatment (P<0.0001), and TMZ pretreatment promoted the nuclear translocation of Nrf2 (P<0.01). Cisplatin significantly promoted the expression of Keap-1 (P<0.0001) and hemo oxygenase 1 (HO-1) (P<0.0001). TMZ pretreatment significantly inhibited the expression of Keap-1 (P<0.0001) and significantly enhanced the expression of HO-1 (P<0.001). Conclusions TMZ significantly inhibited the myocardial injury induced by cisplatin, in part owing to the Keap-1/Nrf2/HO-1 associated antioxidant defense system. The findings suggest that TMZ has a cardioprotective effect as an antioxidant for the treatment of myocardial injury induced by chemotherapeutic drugs. -
Key words:
- cisplatin /
- trimetazidine /
- oxidative stress /
- Nrf2 /
- Keap-1 /
- hemo oxygenase 1(HO-1)
-
表 1 TMZ明显减低Keap-1和Nrf2蛋白的表达,增强HO-1的表达
组别 Keap-1 Nrf2 HO-1 对照组 1.00±0.02 1.00±0.09 1.00±0.01 Cisplatin 1.20±0.05** 1.38±0.04*** 1.14±0.03** Cisplatin+TMZ 1.03±0.05## 1.12±0.02## 1.33±0.05## 与对照组相比,**P<0.01和***P<0.001;与顺铂组相比,##P<0.01 表 2 TMZ通过抑制Keap-1调控Nrf2及HO-1的表达
组别 Keap-1 Nrf2 HO-1 对照组 1.00±0.02 1.00±0.13 1.00±0.17 Cisplatin 2.33±0.07**** 2.97±0.32**** 6.89±0.73**** Cisplatin+TMZ 1.83±0.06#### 2.01±0.21## 10.43±0.33### 与对照组相比,****P<0.0001;与顺铂组相比,分别为##P<0.01、###P<0.001和####P<0.0001 -
[1] Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action[J]. Eur J Pharmacol, 2014, 740:364-378. doi: 10.1016/j.ejphar.2014.07.025 [2] El-Awady El-SE, Moustafa YM, Abo-Elmatty DM, et al. Cisplatin-induced cardiotoxicity: mechanisms and cardioprotective strategies[J]. Eur J Pharmacol, 2011, 650(1):335-341. doi: 10.1016/j.ejphar.2010.09.085 [3] Patanè S. Cardiotoxicity: cisplatin and long-term cancer survivors[J]. Int J Cardiol, 2014, 175(1):201-202. doi: 10.1016/j.ijcard.2014.04.238 [4] Kalam K, Marwick TH. Role of cardioprotective therapy for prevention of cardiotoxicity with chemotherapy: a systematic review and meta-analysis[J]. Eur J Cancer, 2013, 49(13):2900-2909. doi: 10.1016/j.ejca.2013.04.030 [5] El-Agamy DS, El-Harbi KM, Khoshhal S, et al. Pristimerin protects against doxorubicin-induced cardiotoxicity and fibrosis through modulation of Nrf2 and MAPK/NF- kB signaling pathways[J]. Cancer Manag Res, 2018, 11:47-61. doi: 10.2147/CMAR.S186696 [6] Shinjo T, Tanaka T, Okuda H, et al. Propofol induces nuclear localization of Nrf2 under conditions of oxidative stress in cardiac H9c2 cells[J]. PLoS One, 2018, 13(4):e0196191. doi: 10.1371/journal.pone.0196191 [7] Hao E, Lang F, Chen Y, et al. Resveratrol alleviates endotoxin-induced myocardial toxicity via the Nrf2 transcription factor[J]. PLoS One, 2013, 8(7):e69452. doi: 10.1371/journal.pone.0069452 [8] Li L, Du J, Lian Y, et al. Protective effects of Co- enzyme Q10 against hydrogen peroxide-induced oxidative stress in PC12 cell: the role of Nrf2 and antioxidant enzymes[J]. Cell Mol Neurobiol, 2016, 36(1):103-111. doi: 10.1007/s10571-015-0224-4 [9] Kabel AM, Elkhoely AA. Ameliorative Effect of Coenzyme Q10 and/ or candesartan on carboplatin-induced nephrotoxicity: roles of apoptosis, transforming growth factor-beta1, nuclear factor Kappa-B and the Nrf2/HO-1 pathway[J]. Asian Pac J Cancer Prev, 2017, 18(6):1629-1636. [10] Onay-Besikci A, Özkan SA. Trimetazidine revisited: a comprehensive review of the pharmacological effects and analytical techniques for the determination of trimetazidine[J]. Cardiovasc Ther, 2008, 26(2):147-165. doi: 10.1111/j.1527-3466.2008.00043.x [11] Molinari F, Pin F, Gorini S, et al. The mitochondrial metabolic reprogramming agent trimetazidine as an 'exercise mimetic' in cachectic C26-bearing mice[J]. J Cachexia Sarcopenia Muscle, 2017, 8(6):954-973. doi: 10.1002/jcsm.12226 [12] Zheng W, Liu C. The cystathionine γ-lyase/hydrogen sulfide pathway mediates the trimetazidine-induced protection of H9c2 cells against hypoxia/reoxygenation-induced apoptosis and oxidative stress[J]. Anatol J Cardiol, 2019, 22(3):102-111. [13] El-Sherbeeny NA, Attia GM. The protective effect of trimetazidine against cisplatin-induced nephrotoxicity in rats[J]. Can J Physiol Pharmacol, 2016, 94(7):745-751. doi: 10.1139/cjpp-2015-0472 [14] Ateyya H, Yosef H, Nader MA. Ameliorative effect of trimetazidine on cisplatin-induced hepatotoxicity in rats[J]. Can J Physiol Pharmacol, 2016, 94(2):225-230. doi: 10.1139/cjpp-2015-0304 [15] Kisaoglu A, Borekci B, Yapca OE, et al. Tissue damage and oxidant/antioxidant balance[J]. Eurasian J Med, 2013, 45(1):47-49. doi: 10.5152/eajm.2013.08 [16] Karthikeyan K, Bai BR, Devaraj SN. Cardioprotective effect of grape seed proanthocyanidins on isoproterenol-induced myocardial injury in rats[J]. Int J Cardiol, 2007, 115(3):326-333. doi: 10.1016/j.ijcard.2006.03.016 [17] Yüce A, Ateşşahin A, Ceribaşi AO, et al. Ellagic acid prevents cisplatin-induced oxidative stress in liver and heart tissue of rats[J]. Basic Clin Pharmacol Toxicol, 2007, 101(5):345-349. doi: 10.1111/j.1742-7843.2007.00129.x [18] Yousef MI, Saad AA, El-Shennawy LK. Protective effect of grape seed proanthocyanidin extract against oxidative stress induced by cisplatin in rats[J]. Food Chem Toxicol, 2009, 47(6):1176-1183. doi: 10.1016/j.fct.2009.02.007 [19] Zhang H, Chen X, Zong B, et al. Gypenosides improve diabetic cardiomyopathy by inhibiting ROS-mediated NLRP3 inflammasome activation[J]. J Cell Mol Med, 2018, 22(9):4437-4448. [20] Tang SG, Liu XY, Wang SP, et al. Trimetazidine prevents diabetic cardiomyopathy by inhibiting Nox2/TRPC3-induced oxidative stress[J]. J Pharmacol Sci, 2019, 139(4):311-318. doi: 10.1016/j.jphs.2019.01.016 [21] Steggall A, Mordi IR, Lang CC. Targeting metabolic modulation and mitochondrial dysfunction in the treatment of heart failure[J]. Diseases, 2017, 5(2):E14. doi: 10.3390/diseases5020014 [22] Reziwan K, Sun D, Zhang B, et al. MicroRNA-1225 activates Keap1-Nrf2-HO-1 signalling to inhibit TNFα-induced osteoclastogenesis by mediating ROS generation[J]. Cell Biochem Funct, 2019, 37:256-265. doi: 10.1002/cbf.3394 [23] Jiang Y, Zhou W, Zhang X, et al. Protective effect of blood cora polysaccharides on H9c2 rat heart cells injury induced by oxidative stress by activating Nrf2/HO-1 signal pathway[J]. Front Nutr, 2021, 8:632161. doi: 10.3389/fnut.2021.632161 [24] Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death[J]. Cell, 2012, 149(5):1060-1072. doi: 10.1016/j.cell.2012.03.042 [25] Stockwell BR, Friedmann Angeli JP, Bayir H, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease[J]. Cell, 2017, 171(2):273-285. doi: 10.1016/j.cell.2017.09.021 [26] Sun XH, Ou ZH, Chen RC, et a1. Activation of the p62-Keapl-Nrf2 pathway protects against ferroptosis in hepatocellular carcinoma cells[J]. Hepatology, 2016, 63(1):173-184. [27] Yang WS, SriRamaratnam R, Welsch ME, et al. Regulation of ferroptotic cancer cell death by GPX4[J]. Cell, 2014, 156(1-2):317-331. doi: 10.1016/j.cell.2013.12.010 [28] Friedmann Angeli JP, Schneider M, Proneth B, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice[J]. Nat Cell Biol, 2014, 16(12):1180-1191. doi: 10.1038/ncb3064 [29] Li J, Cao F, Yin HL, et al. Ferroptosis: past, present and future[J]. Cell Death Dis, 2020, 11(2):88. doi: 10.1038/s41419-020-2298-2 [30] Shin D, Kim EH, Lee J, et al. Nrf2 inhibition reverses resistance to GPX4 inhibitor-induced ferroptosis in head and neck cancer[J]. Free Radic Biol Med, 2018, 129:454-462. doi: 10.1016/j.freeradbiomed.2018.10.426 -




 下载:
下载: