Clinical study of the effect of intestinal flora alteration induced by appendectomy on prognosis of colorectal cancer
-
摘要:
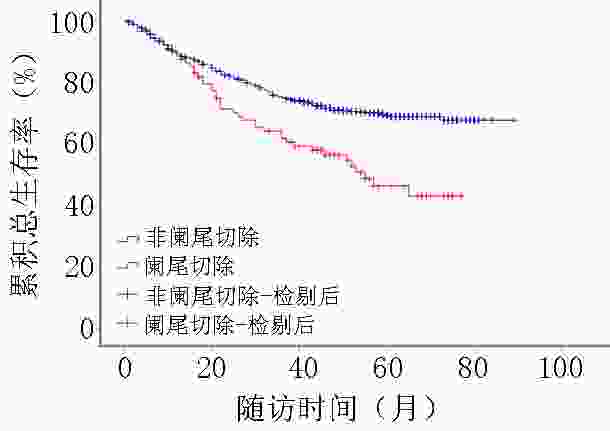
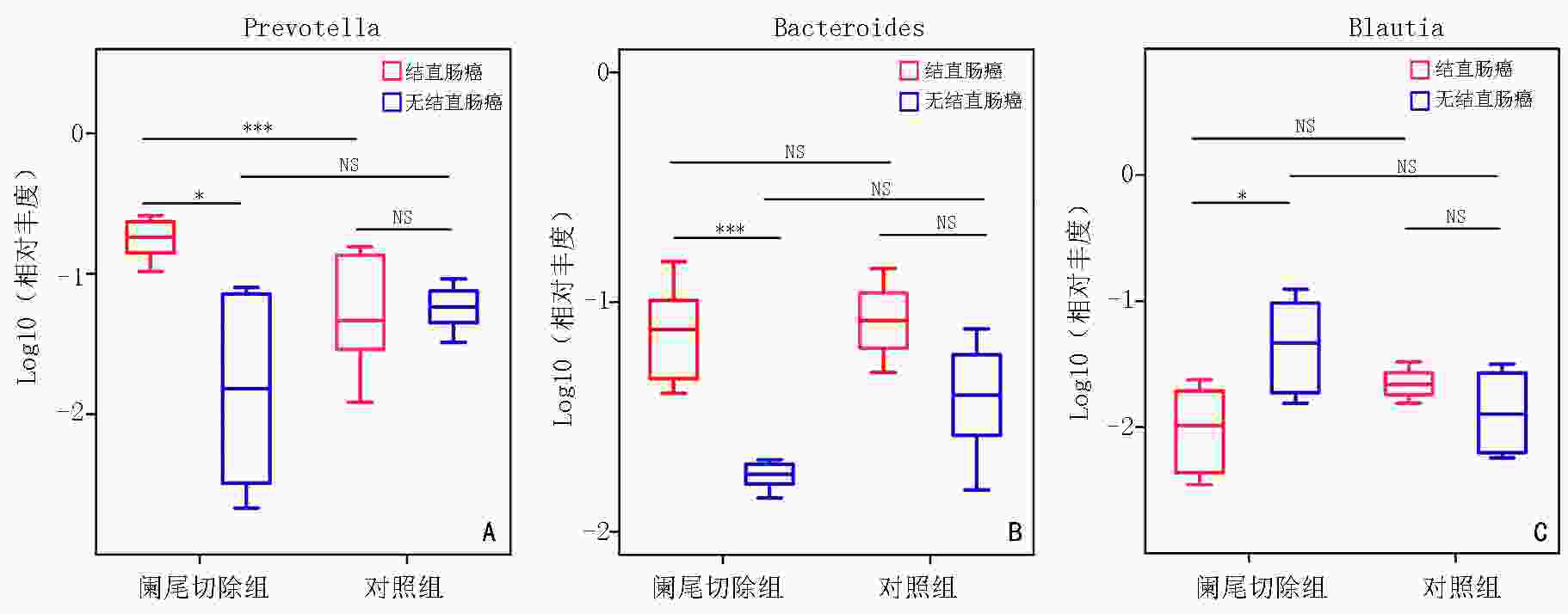
目的 探究既往阑尾切除对结直肠癌预后的影响及可能存在机制。 方法 回顾性收集2013年1月至2016年12月西安交通大学第一附属医院收治的763例结直肠癌患者的临床及病理信息,根据肿瘤诊断前是否存在阑尾切除史,分为阑尾切除组(89例)和非阑尾切除组(674例),分析比较两组患者的总体生存率,以及既往阑尾切除史与结直肠癌患者预后的关系。应用阑尾切除后结直肠癌诱导小鼠模型,通过实时荧光定量聚合酶链式反应(qPCR)比较其粪便中拟杆菌属(Bacteroides)、普氏菌属(Prevotella)、布氏菌属(Blautia)相对丰度。 结果 阑尾切除组患者的3、5年总体生存率均明显低于非阑尾切除组(61.8% vs. 75.1%,46.4% vs. 69.3%),差异具有统计学意义(χ2=13.777,P<0.05);阑尾切除是结直肠癌预后独立危险因素(HR=1.475,95%CI:1.030~2.114)。阑尾切除后行氧化偶氮甲烷(AOM)+ 葡聚糖硫酸钠(DSS)诱导发生结直肠癌的小鼠,其粪便中显著富集拟杆菌属、普氏菌属,而布氏菌属丰度明显降低。 结论 阑尾切除可能是结直肠癌不良预后的独立危险因素,其潜在机制和阑尾切除导致某些致癌致炎细菌在肠道中富集相关。 Abstract:Objective To assess the association between appendectomy and prognosis of secondary colorectal cancer. Methods We retrospectively reviewed the data of 763 patients with colorectal cancer in The First Affiliated Hospital of Xi'an Jiaotong University from January 2013 to December 2016. Based on the inclusion and exclusion criteria, 89 patients who underwent appendectomy were included in the appendectomy group and 674 patients who did not undergo appendectomy were included in the non-appendectomy group. Overall survival rates were compared between the two groups, and the association between appendectomy and prognosis prediction was evaluated. Using the murine appendectomy model of chronic colitis-associated colorectal cancer, the relative abundance of Bacteroides, Prevotella, and Blautia in the feces of mice was compared using real-time fluorescent quantitative polymerase chain reaction (qPCR). Results The 3- and 5-year overall survival rates were significantly lower in the appendectomy group than in the non-appendectomy group (61.8% vs. 75.1% and 46.4% vs. 69.3%, respectively), and the differences between the two groups were statistically significant (χ2=13.777, P<0.05). The analysis showed that appendectomy was an independent prognostic risk factor for colorectal cancer (HR=1.475, 95%CI: 1.030~2.114). The abundance of Prevotella and Bacteroides was higher and that of Blautia was lower in the feces of mice with colorectal cancer induced by azoxymethane (AOM)+ dextran sulfate sodium (DSS) after appendectomy. Conclusions Appendectomy may be an independent risk factor for poor prognosis of colorectal cancer, and its potential mechanism is related to the enrichment of some inflammation-related bacteria in gut flora structure caused by appendectomy. -
Key words:
- colorectal cancer /
- appendectomy /
- prognosis /
- gut bacteria
-
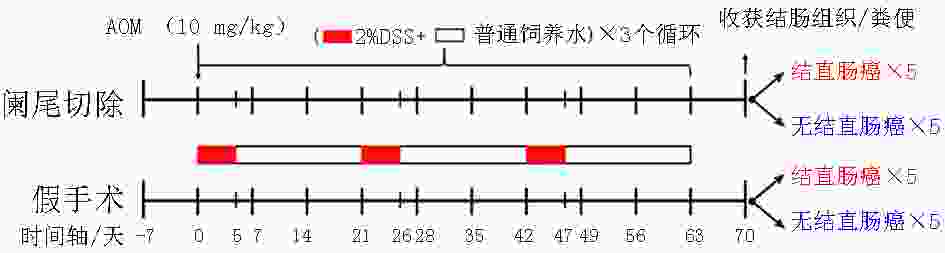
图 1 小鼠阑尾切除后结直肠癌诱导模型制作流程图[11]
表 1 阑尾切除组与非阑尾切除组基本临床资料
例(%) 临床基线资料 阑尾切除组(89例) 非阑尾切除组(674例) χ2/Z P 性别(例) χ2=0.430 0.521 男 52(58.4) 369(54.7) 女 37(41.6) 305(45.3) 年龄(岁) χ2=0.193 0.660 <65 50(56.2) 362(53.7) ≥65 39(43.8) 312(46.3) 吸烟 Z=−0.310 0.757 无 64(1.9) 478(70.9) 有 18(20.2) 152(22.6) 已戒烟 7(7.9) 44(6.5) 饮酒 Z=−0.259 0.796 无 82(92.1) 626(92.9) 有 7(7.9) 48(7.1) 糖尿病史 χ2=1.648 0.199 无 77(86.5) 612(90.8) 有 12(13.5) 62(9.2) T分期(期) χ2=0.092 0.762 T1~T2 12(13.5) 99(14.7) T3~T4 77(86.5) 575(85.3) 淋巴结转移 Z=−1.413 0.158 阴性 47(52.8) 392(58.2) 阳性 35(39.3) 272(40.3) 无法评价 7(7.9) 10(1.5) 远处转移 χ2=2.535 0.111 有 4(4.5) 65(9.6) 无 85(95.5) 609(90.4) 临床分期(期) χ2=0.658 0.417 Ⅰ~Ⅱ 46(51.7) 379(56.2) Ⅲ~Ⅳ 43(48.3) 295(43.8) R0切除 χ2=1.394 0.238 否 12(13.5) 64(9.5) 是 77(86.5) 610(90.5) 术后放/化疗 χ2=1.180 0.277 否 57(64.0) 391(58.0) 是 32(36.0) 283(42.0) 表 2 不同因素对于结直肠癌总生存率影响的Cox单因素与多因素回归分析
因素 例数(%)
(n=763)单因素分析 多因素分析 风险比 P 风险比 P 年龄(岁) <0.001 <0.001 <65 412(54.0) 1 1 ≥65 351(46.0) 2.378(1.746~3.238) 2.102(1.533~2.882) 性别 0.943 0.763 男 421(55.2) 1 1 女 342(44.8) 1.051(0.811~1.361) 1.042(0.795~1.367) 阑尾切除 <0.001 0.034 否 674(88.3) 1 1 是 89(11.7) 2.023(1.392~2.939) 1.475(1.030~2.114) 吸烟 0.100 无 542(71.0) 1 有 170(22.3) 0.915(0.292~2.865) 戒烟 51(6.7) 1.305(0.830~2.053) 饮酒 0.740 无 708(92.8) 1 有 55(7.2) 0.681(0.302~2.606) 糖尿病 0.004 0.151 无 689(90.3) 1 1 有 74(9.7) 1.724(1.193~2.493) 1.321(0.904~1.931) 临床分期 <0.001 0.003 Ⅰ~Ⅱ 425(55.7) 1 1 Ⅲ~Ⅳ 338(44.3) 4.355(3.257~5.823) 3.091(1.460~6.546) T分期 <0.001 0.931 T1~T2 111(14.5) 1 1 T3~T4 652(85.5) 1.744(1.124~2.706) 0.979(0.611~1.571) 淋巴结转移 <0.001 0.789 阴性 439(57.5) 1 1 阳性 307(40.3) 3.321(2.507~4.399) 0.992(0.508~1.939) 无法评价 17(2.2) 10.321(5.990~17.784) 1.231(0.546~2.775) 远处转移 <0.001 0.001 无 694(91.0) 1 1 有 69(9.0) 5.034(3.691~6.864) 1.985(1.324~2.975) 分化程度 <0.001 <0.001 高 39(5.1) 1 1 中 606(79.4) 1.828(0.809~4.129) 1.525(0.663~3.509) 低 118(15.5) 4.568(1.976~10.564) 2.779(1.172~6.590) 肿瘤位置 0.025 0.613 近端结肠(盲肠-脾曲) 162(21.2) 1 1 远端结肠(降结肠-乙状结肠) 139(18.2) 0.843(0.572~1.240) 0.817(0.547~1.220) 直肠(直乙交界-直肠) 462(60.6) 0.661(0.486~0.899) 0.914(0.658~1.269) R0切除 <0.001 0.002 否 76(10.0) 1 1 是 687(90.0) 0.199(0.148~0.268) 0.534(0.357~0.799) 术后放/化疗 <0.001 <0.001 否 448(58.7) 1 1 是 315(41.3) 0.609(0.464~0.799) 0.590(0.446~0.781) 表 3 阑尾切除暴露时间与结直肠癌总生存率相关关系的Cox单因素与多因素回归分析
因素 例数(%)
(n=763)单因素分析 多因素分析* 风险比 P 风险比 P 阑尾切除时间(年) 0.002 0.025 无 674(88.4) 1 1 1~3 10(1.3) 2.649(1.245~5.635) 2.772(1.272~6.043) 3~10 10(1.3) 1.301(0.483~3.503) 0.828(0.301~2.277) ≥10 69(9.0) 1.827(1.256~2.659) 1.414(0.961~2.080) *:根据年龄、性别、肿瘤分化、肿瘤临床分期、肿瘤位置、R0切除、术后放/化疗、糖尿病进行校正 -
[1] Dekker E, Tanis P J, Vleugels J L A, et al. Colorectal cancer[J]. The Lancet, 2019, 394(10207):1467-1480. doi: 10.1016/S0140-6736(19)32319-0 [2] 王锡山.中美结直肠癌流行病学特征及防诊治策略的对比分析[J].中华结直肠疾病电子杂志,2017,6:447-453. doi: 10.3877/cma.j.issn.2095-3224.2017.06.002 [3] 于君,杨佳.肠道微生态与结直肠癌: 基础和临床转化研究进展[J].中华消化杂志,2020,40(12):793-796. doi: 10.3760/cma.j.cn311367-20200907-00537 [4] Mcvay JRJ. The appendix in relation to neoplastic disease[J]. Cancer, 1964, 17:929-937. doi: 10.1002/1097-0142(196407)17:7<929::AID-CNCR2820170713>3.0.CO;2-O [5] Lai HW, Loong CC, Tai LC, et al. Incidence and odds ratio of appendicitis as first manifestation of colon cancer: a retrospective analysis of 1873 patients[J]. J Gastroenterol Hepatol, 2006, 21(11):1693-1696. [6] Sánchez-Alcoholado L, Fernández-García J, Gutiérrez-Repiso C, et al. Incidental prophylactic appendectomy is associated with a profound microbial dysbiosis in the long-term[J]. Microorganisms, 2020, 8(4):609. doi: 10.3390/microorganisms8040609 [7] Mohamed I, Chan S, Bhangu A, et al. Appendicitis as a manifestation of colon cancer: should we image the colon after appendicectomy in patients over the age of 40 years[J]? Int J Colorectal Dis, 2019, 34(3):527-531. [8] Wong SH, Kwong TNY, Chow TC, et al. Quantitation of faecal Fusobacterium improves faecal immunochemical test in detecting advanced colorectal neoplasia[J]. Gut, 2017, 66(8):1441-1448. doi: 10.1136/gutjnl-2016-312766 [9] Helsingen LM, Vandvik PO, Jodal HC, et al. Colorectal cancer screening with faecal immunochemical testing, sigmoidoscopy or colonoscopy: a clinical practice guideline[J]. BMJ, 2019, 367:1-16. [10] Fritz A, Percy C, Jack A, et al. International classification of diseases for oncology (ICD-O)[M]. Geneva: WHO Press, 2013:147. [11] Li Y, Liu J, Liu G, et al. Murine appendectomy model of chronic colitis associated colorectal cancer by precise localization of caecal patch[J]. J Vis Exp, 2019, 150:e59921. [12] Vitetta L, Chen J, Clarke S. The vermiform appendix: an immunological organ sustaining a microbiome inoculum[J]. Clin Sci (Lond), 2019, 133(1):1-8. doi: 10.1042/CS20180956 [13] Killinger BA, Madaj Z, Sikora JW, et al. The vermiform appendix impacts the risk of developing Parkinson's disease[J]. Sci Transl Med, 2018, 10(465):eaar5280. doi: 10.1126/scitranslmed.aar5280 [14] Mellemkjaer L, Johansen C, Linet MS, et al. Cancer risk following appendectomy for acute appendicitis (Denmark)[J]. Cancer Causes Control, 1998, 9(2):183-187. doi: 10.1023/A:1008834311514 [15] Gao ZG, Guo BM, Gao RY, et al. Microbiota disbiosis is associated with colorectal cancer[J]. Front Microbiol, 2015, 6:20. [16] Flemer B, Warren RD, Barrett MP, et al. The oral microbiota in colorectal cancer is distinctive and predictive[J]. Gut, 2018, 67(8):1454-1463. doi: 10.1136/gutjnl-2017-314814 [17] Rozek LS, Schmit SL, Greenson JK, et al. Tumor-infiltrating lymphocytes, crohn's-like lymphoid reaction, and survival from colorectal cancer[J]. J Natl Cancer Inst, 2016, 108(8):djw027. [18] Donaldson GP, Ladinsky MS, Yu KB, et al. Gut microbiota utilize immunoglobulin A for mucosal colonization[J]. Science, 2018, 360(6390):795-800. doi: 10.1126/science.aaq0926 [19] 朱小坚,骆晨,朱正明.肠道微生态与结直肠癌关系的研究进展[J].中华消化杂志,2019,39(7):496-498. doi: 10.3760/cma.j.issn.0254-1432.2019.07.014 [20] Masahata K, Umemoto E, Kayama H, et al. Generation of colonic IgA-secreting cells in the caecal patch[J]. Nat Commun, 2014, 5:3704. doi: 10.1038/ncomms4704 -



 下载:
下载: