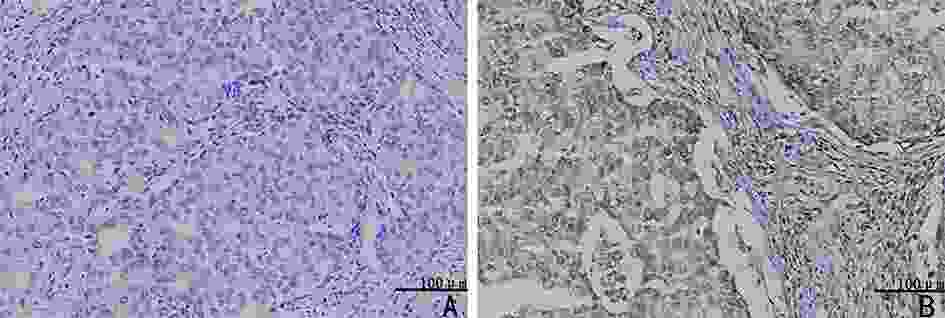
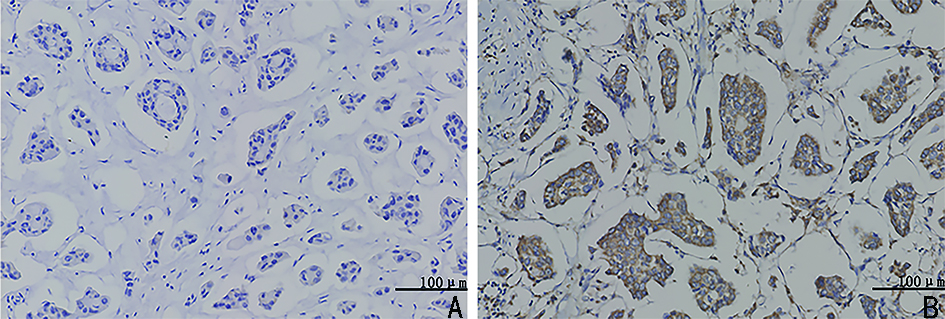
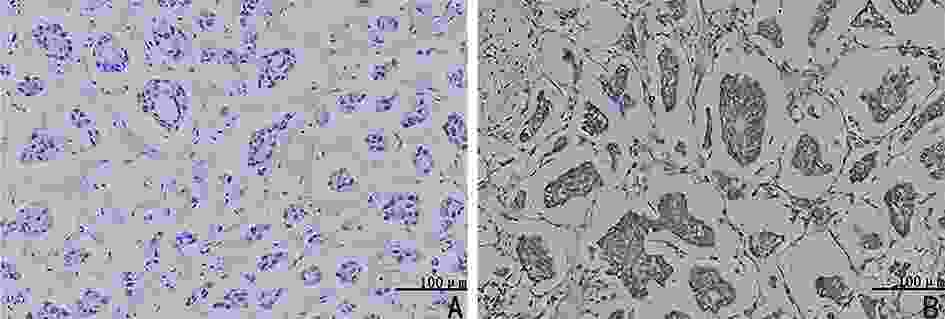
Study on the relationship between acetaldehyde dehydrogenase 2 and neoadjuvant chemotherapy for invasive micropapillary carcinoma of breast
-
摘要:
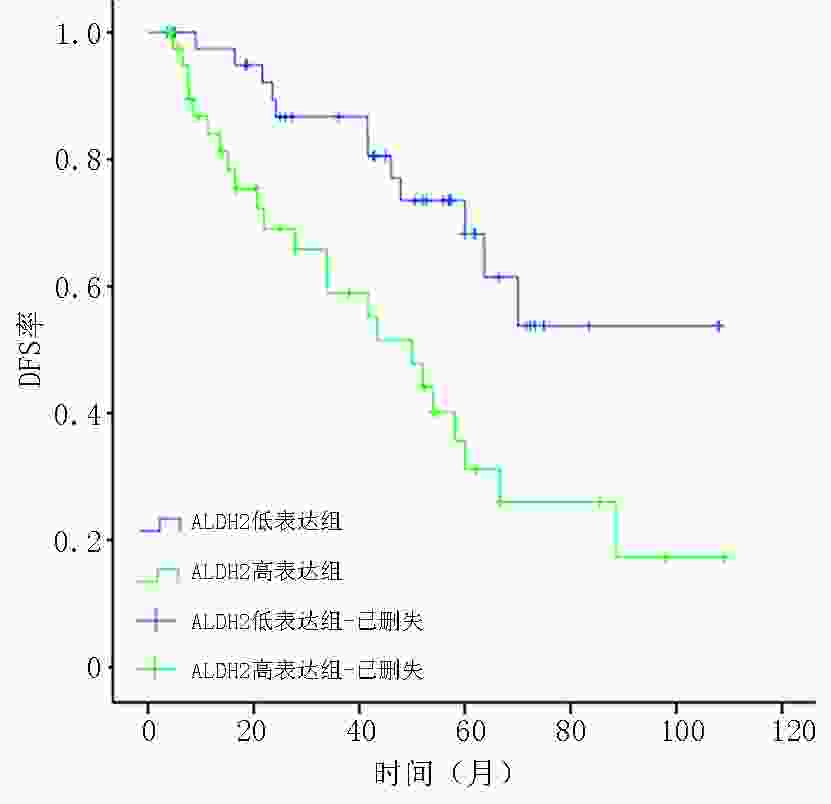
目的 检测乳腺浸润性微乳头状癌(invasive micropapillary carcinoma,IMPC)中乙醛脱氢酶2(acetaldehyde dehydrogenase 2,ALDH2)的表达,探讨ALDH2表达水平对IMPC患者新辅助化疗方案的指导意义。 方法 选取2009年1月至2014年5月298例于天津医科大学肿瘤医院行新辅助化疗(neoadjuvant chemotherapy,NAC)的乳腺癌患者的临床病理资料,其中IMPC为84例、浸润性导管癌非特殊型(invasive ductal carcinoma no special type,IDC-NOS)为214例。采用倾向评分匹配法成功匹配84例IMPC与IDC-NOS,检测ALDH2在IMPC组和IDC-NOS组中的表达差异,分析IMPC患者中ALDH2不同表达组的临床病理特征、NAC反应分级与预后相关性。 结果 ALDH2在IMPC中的表达水平为48.8%(41/84),显著高于IDC-NOS的33.3%(28/84),两者比较差异具有统计学意义(P=0.041);IMPC患者中ALDH2表达水平与N分期(rs=0.348,P=0.017)及p53突变水平(rs=0.262,P=0.016)呈正相关,与NAC反应分级及无病生存率(disease-free survival,DFS)呈负相关(均P<0.05)。 结论 在IMPC中ALDH2高表达且与NAC反应分级及DFS呈负相关。针对ALDH2高表达的IMPC患者应尽早行手术切除,以延长生存预后。 Abstract:Objective To detect the expression of acetaldehyde dehydrogenase 2 (ALDH2) in invasive micropapillary carcinoma (IMPC) and explore the significance of ALDH2 expression levels in guiding neoadjuvant chemotherapy regimen for patients with IMPC of breast. Methods Clinicopathological data of 298 patients with breast cancer who underwent neoadjuvant chemotherapy (NAC) at Tianjin Medical University Tumor Hospital from January 2009 to May 2014 were assessed. Of the 298 patients, 84 with IMPC and 214 with invasive ductal carcinoma of no special type (IDC-NOS). Eighty-four patients with IMPC and IDC-NOS were successfully matched by the propensity score matching method. Difference in the expression of ALDH2 between the IMPC and IDC-NOS groups was detected, and clinicopathological features, NAC response grade, and prognosis of patients with IMPC in different ALDH2 expression groups were analyzed. Results The expression level of ALDH2 in IMPC was 48.8% (41/84), which was significantly higher than that in IDC-NOS (33.3%, 28/84), and the difference was statistically significant (P=0.041). In the IMPC group, the expression level of ALDH2 was positively correlated with N stage (rs=0.348, P=0.017) and p53 mutation level (rs=0.262, P=0.016) and negatively correlated with the grade of NAC response and disease-free survival (DFS) (all P<0.05). Conclusions ALDH2 was highly expressed in IMPC and was negatively correlated with NAC response grade and DFS. IMPC patients with high ALDH2 expression should be surgically treated as soon as possible to prolong survival. -
表 1 IMPC与IDC-NOS临床病理学特征及NAC反应分级的比较
相关参数 IMPC组(n=84) IDC-NOS组(n=84) χ2 P 年龄(岁) 0.024 0.876 ≤50 47(56.0) 48(57.1) >50 37(44.0) 36(42.9) NAC后T分期(期) 1.887 0.389 T1 13(15.5) 13(15.4) T2 39(46.4) 47(56.0) T3 32(38.1) 24(28.6) NAC后N分期(期) N0 7(8.3) 13(15.5) 8.323 0.040 N1 20(23.8) 15(17.9) N2 15(17.9) 27(32.1) N3 42(50.0) 29(34.5) 组织学分级(级) 12.979 0.002 Ⅰ 8(9.5) 5(6.0) Ⅱ 51(60.7) 71(84.5) Ⅲ 25(29.8) 8(9.5) 淋巴管癌栓 0.137 0.711 无 16(32.7) 11(28.9) 有 33(67.3) 27(71.1) ER 9.033 0.003 阴性 13(15.5) 30(35.7) 阳性 71(84.5) 54(64.3) PR 14.374 <0.001 阴性 21(25.0) 45(53.6) 阳性 63(75.0) 39(46.4) HER-2 1.025 0.311 阴性 56(66.7) 62(73.8) 阳性 28(33.3) 22(26.2) Ki-67 0.001 1.000 低表达 32(38.0) 32(38.0) 高表达 52(62.0) 52(62.0) p53突变 0.226 0.635 低表达 50(59.5) 53(63.1) 高表达 34(40.5) 31(36.9) 分子分型 25.834 <0.001 Luminal A 16(19.0) 26(31.0) Luminal B 57(67.9) 28(33.3) HER-2过表达型 8(9.5) 9(10.7) TNBC 3(3.6) 21(25.0) ALDH2表达水平 4.156 0.041 低表达 43(51.2) 56(66.7) 高表达 41(48.8) 28(33.3) NAC反应分级(级) 30.661 <0.001 0 38(45.2) 22(26.2) Ⅰ 44(52.4) 32(38.1) Ⅱ 2(2.4) 30(35.7) ()内单位为% 表 2 ALDH2与IMPC患者的临床病理特征及NAC反应分级的相关性
相关参数 ALDH2低表达组(n=43) ALDH2高表达组(n=41) χ2 P 年龄(岁) 3.186 0.074 ≤50 20(46.5) 27(65.9) >50 23(53.5) 14(34.1) NAC后T分期(期) 3.903 0.142 T1 8(18.6) 5(12.2) T2 23(53.5) 16(39.0) T3 12(27.9) 20(48.8) NAC后N分期(期) 10.196 0.017 N0 3(7.0) 4(9.7) N1 15(34.9) 5(12.2) N2 10(23.2) 5(12.2) N3 15(34.9) 27(65.9) 组织学分级(级) 2.974 0.226 Ⅰ 2(4.6) 6(14.6) Ⅱ 26(60.5) 25(61.0) Ⅲ 15(34.9) 10(24.4) 淋巴管癌栓 1.238 0.266 无 9(40.9) 7(25.9) 有 13(59.1) 20(74.1) ER 2.567 0.109 阴性 4(9.3) 9(22.0) 阳性 39(90.7) 32(78.0) PR 1.922 0.166 阴性 8(18.6) 13(31.7) 阳性 35(81.4) 28(68.3) HER-2 0.095 0.758 阴性 28(65.1) 28(68.3) 阳性 15(34.9) 13(31.7) Ki-67 0.077 0.825* 低表达 17(39.5) 15(36.6) 高表达 26(60.5) 26(63.4) p53突变 5.777 0.016 低表达 31(72.1) 19(46.3) 高表达 12(27.9) 22(53.7) 分子分型 3.055 0.383 Luminal A 11(25.6) 5(12.2) Luminal B 28(65.1) 29(70.7) HER-2过表达型 3(7.0) 5(12.2) TNBC 1(2.3) 2(4.9) NAC反应分级(级) 9.020 0.011 0 13(30.2) 25(61.0) Ⅰ 28(65.1) 16(39.0) Ⅱ 2(4.7) 0 ()内单位为%;* :Fisher确切检验结果 表 3 IMPC患者DFS的单因素生存分析
相关参数 例数(n=84) DFS率 χ2 P 年龄(岁) 0.007 0.931 ≤50 47 27(57.4) >50 37 22(59.5) NAC后T分期(期) 6.122 0.047 T1 13 10(76.9) T2 39 25(64.1) T3 32 14(43.8) NAC后N分期(期) N0 7 6(85.7) 13.842 0.003 N1 20 12(60.0) N2 15 13(86.7) N3 42 18(42.9) 组织学分级(级) 0.321 0.852 Ⅰ 8 5(62.5) Ⅱ 51 30(58.8) Ⅲ 25 14(56.0) 淋巴管癌栓 3.131 0.077 无 16 12(75.0) 有 33 19(57.6) ER 0.043 0.835 阴性 13 7(53.8) 阳性 71 42(59.2) PR 1.878 0.171 阴性 21 9(42.9) 阳性 63 40(63.5) HER-2 0.013 0.910 阴性 56 32(57.1) 阳性 28 17(60.7) Ki-67 1.290 0.256 低表达 32 19(59.4) 高表达 52 30(57.7) p53突变 10.094 0.001 低表达 50 35(70.0) 高表达 34 14(41.2) 分子分型 3.655 0.301 Luminal A 16 12(75.0) Luminal B 57 31(54.4) HER-2过表达型 8 3(37.5) TNBC 3 3(100.0) ALDH2表达水平 8.651 0.003 低表达 43 31(72.1) 高表达 41 18(43.9) NAC反应分级(级) 0.025 0.988 0 38 22(57.9) Ⅰ 44 26(59.1) Ⅱ 2 1(50.0) ()内单位为% 表 4 IMPC患者DFS的Cox比例风险回归模型分析
相关参数 HR 95%CI P NAC后T分期(T1 vs. T2 vs. T3) 1.451 0.731~2.880 0.287 NAC后N分期(N0 vs. N1 vs. N2 vs. N3) 1.472 0.948~2.284 0.085 p53突变(低表达 vs. 高表达) 2.735 1.374~5.442 0.004 ALDH2(低表达 vs. 高表达) 2.607 1.284~5.294 0.008 -
[1] Song Y, Sun H, Wu K, et al. sLe(x) expression in invasive micropapillary breast carcinoma is associated with poor prognosis and can be combined with MUC1/EMA as a supplementary diagnostic indicator[J]. Cancer Biol Med, 2021, 18(2):477-489. doi: 10.20892/j.issn.2095-3941.2020.0422 [2] Alvarado-Cabrero I, Alderete-Vazquez G, Quintal-Ramirez M, et al. Incidence of pathologic complete response in women treated with preoperative chemotherapy for locally advanced breast cancer: correlation of histology, hormone receptor status, Her2/Neu, and gross pathologic findings[J]. Ann Diagn Pathol, 2009, 13(3):151-157. doi: 10.1016/j.anndiagpath.2009.02.003 [3] Toledo-Guzman ME, Hernandez MI, Gomez-Gallegos AA, et al. ALDH as a stem cell marker in solid tumors[J]. Curr Stem Cell Res Ther, 2019, 14(5):375-388. doi: 10.2174/1574888X13666180810120012 [4] Ye F, Yu P, Li N, et al. Prognosis of invasive micropapillary carcinoma compared with invasive ductal carcinoma in breast: A meta-analysis of PSM studies[J]. Breast, 2020, 51:11-20. [5] Kurosumi M, Akashi-Tanaka S, Akiyama F, et al. Histopathological criteria for assessment of therapeutic response in breast cancer (2007 version)[J]. Breast Cancer, 2008, 15(1):5-7. doi: 10.1007/s12282-007-0016-x [6] Sun C, Shi L, Gu Y, et al. Clinical effects of neoadjuvant chemotherapy in treating breast cancer[J]. Cancer Biother Radiopharm, 2021, 36(2):174-179. doi: 10.1089/cbr.2019.3545 [7] Goto W, Kashiwagi S, Takada K, et al. Significance of intrinsic breast cancer subtypes on the long-term prognosis after neoadjuvant chemotherapy[J]. J Transl Med, 2018, 16(1):307. doi: 10.1186/s12967-018-1679-0 [8] Li W, Liu F, Lei T, et al. The clinicopathological significance of CD44(+)/CD24(-)/low and CD24(+) tumor cells in invasive micropapillary carcinoma of the breast[J]. Pathol Res Pract, 2010, 206(12):828-834. [9] Najafi M, Mortezaee K, Majidpoor J. Cancer stem cell (CSC) resistance drivers[J]. Life Sci, 2019, 234:116781. doi: 10.1016/j.lfs.2019.116781 [10] Ferreira-Teixeira M, Parada B, Rodrigues-Santos P, et al. Functional and molecular characterization of cancer stem-like cells in bladder cancer: a potential signature for muscle-invasive tumors[J]. Oncotarget, 2015, 6(34):36185-36201. doi: 10.18632/oncotarget.5517 [11] Ramakrishnan S, Granger V, Rak M, et al. Inhibition of EZH2 induces NK cell-mediated differentiation and death in muscle-invasive bladder cancer[J]. Cell Death Differ, 2019, 26(10):2100-2114. doi: 10.1038/s41418-019-0278-9 [12] Wang NN, Wang LH, Li Y, et al. Targeting ALDH2 with disulfiram/copper reverses the resistance of cancer cells to microtubule inhibitors[J]. Exp Cell Res, 2018, 362(1):72-82. [13] Wang LS, Wu ZX. ALDH2 and cancer therapy[J]. Adv Exp Med Biol, 2019, 1193:221-228. [14] Kim J, Chen CH, Yang J, et al. Aldehyde dehydrogenase 2*2 knock-in mice show increased reactive oxygen species production in response to cisplatin treatment[J]. J Biomed Sci, 2017, 24(1):33. doi: 10.1186/s12929-017-0338-8 [15] Komarova TV, Sheshukova EV, Kosobokova EN, et al. The biological activity of bispecific trastuzumab/pertuzumab plant biosimilars may be drastically boosted by disulfiram increasing formaldehyde accumulation in cancer cells[J]. Sci Rep, 2019, 9(1):16168. doi: 10.1038/s41598-019-52507-9 -



 下载:
下载: