Clinical analysis of immunotherapy for advanced gastric/gastroesophageal junction adenocarcinoma
-
摘要:
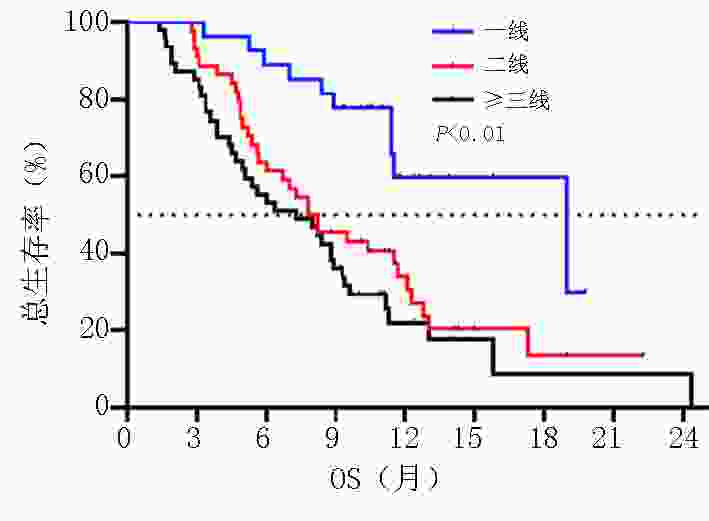
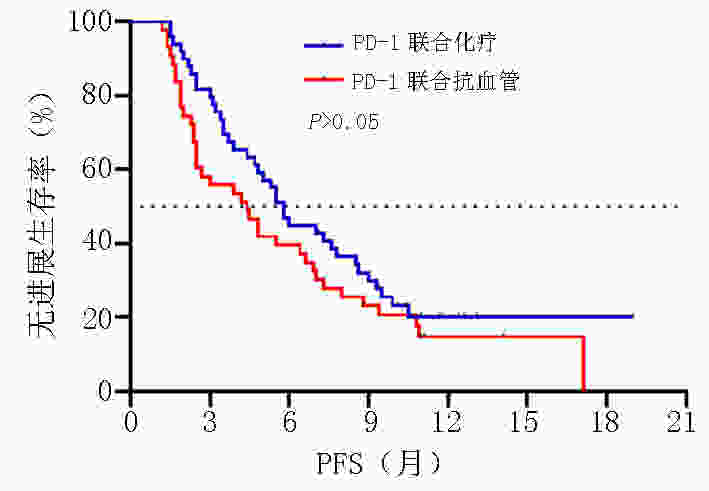
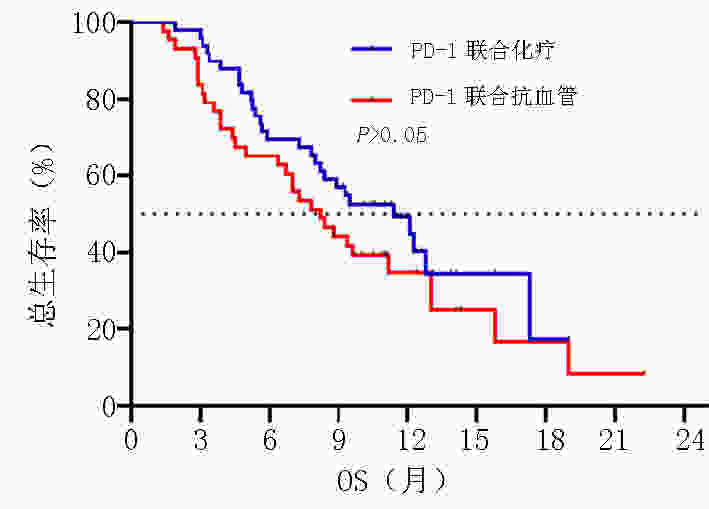
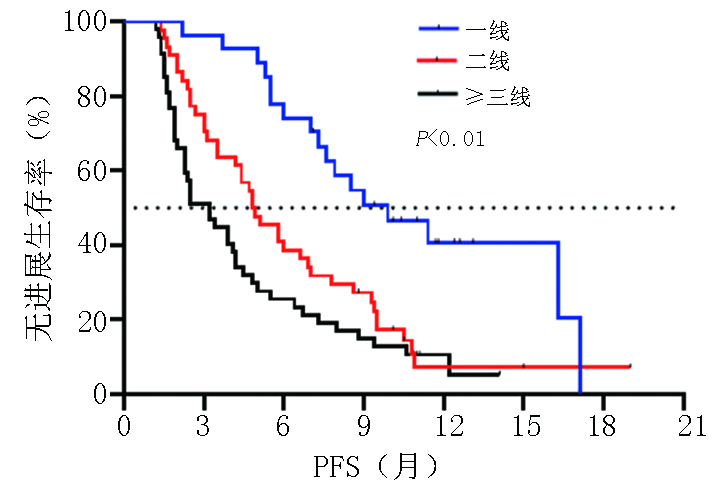
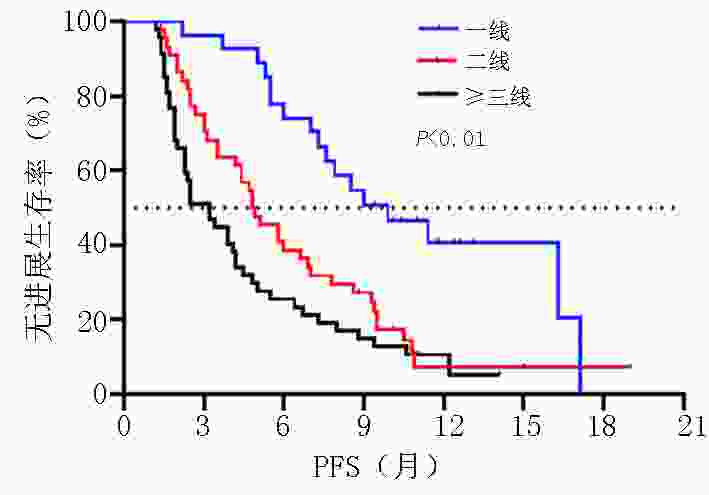
目的 探讨晚期胃/食管胃结合部(gastric/gastroesophageal junction,G/GEJ)腺癌中程序性细胞死亡受体(programmed cell death protein 1, PD-1)单抗临床疗效分析。 方法 收集2018年9月至2020年9月就诊于河南省肿瘤医院接受PD-1单抗治疗晚期胃/食管胃结合部腺癌患者的临床数据资料。 结果 收集123例(5例失访)患者,中位随访时间12.6个月。118例患者客观缓解率(overall response rate, ORR)为22.0%,疾病控制率(disease control rate, DCR)为51.7%,中位无进展生存期(progression-free survival, PFS)为5.0个月,中位总生存期(overall survival, OS)为8.9个月。一线 vs. 二线 vs. 三线及以上ORR为(37.0% vs. 20.5% vs. 14.9%,P=0.002),DCR为(81.5% vs. 54.5% vs. 29.8%,P<0.001),中位PFS为(9.9个月 vs. 4.8个月 vs. 3.2个月),中位OS为(19.0 个月vs. 7.8个月 vs. 7.3个月)。PD-1单抗联合化疗 vs. 抗血管 vs. 化疗及抗血管治疗中位PFS为(5.8个月 vs. 4.4个月 vs. 5.0个月)及中位OS为(11.4个月 vs. 8.2个月 vs. 8.2个月)。免疫联合化疗一线(22例)vs.二线(20例)中位PFS为(9.0个月 vs. 4.7个月,P=0.003),中位OS为(NR vs. 7.8个月,P=0.007)。程序性死亡配体(programmed cell death-ligand 1, PD-L1)表达(CPS≥1% vs. CPS<1%)中ORR为(37.1% vs. 13.3%),DCR为(65.7% vs. 46.7%),中位PFS为(5.8个月 vs. 4.7个月),中位OS为(11.3个月 vs. 9.3个月)。多因素分析显示年龄、ECOG评分、转移灶数目、腹膜转移及免疫治疗线数是患者OS独立影响因素(P<0.05),且年龄为保护性因素(HR=0.498,95%CI:0.255~0.974)。 结论 晚期胃/食管胃结合部腺癌早期采用PD-1单抗联合治疗可有效延长患者PFS及OS。PD-1单抗联合化疗及抗血管治疗均有一定优势,但具体时机尚需进一步探索研究。 Abstract:Objective To evaluate the clinical efficacy of programmed cell death protein 1 (PD-1) antibody for advanced gastric/gastroesophageal junction (G/GEJ) adenocarcinoma. Methods Clinical data of patients with advanced G/GEJ adenocarcinoma treated with PD-1 monoclonal antibody at Henan Cancer Hospital were collected from September 2018 to September 2020. Results Overall, 123 patients were examined, among whom 5 were lost to follow-up. The median follow-up time was 12.6 months. In 118 patients, the objective response rate (ORR) was 22.0%, the disease control rate (DCR) was 51.7%, median progression-free survival (PFS) was 5.0 months, and median overall survival (OS) was 8.9 months. The ORRs to first-, second-, and third-line treatment and beyond were 37.0%, 20.5%, and 14.9%, respectively (P=0.002); the DCRs were 81.5%, 54.5%, and 29.8%, respectively (P<0.001); the median PFS were 9.9, 4.8, and 3.2 months, respectively; and the median OS were 19.0, 7.8, and 7.3 months, respectively. After receiving anti-PD-1 therapy combined with chemotherapy, anti-vascular, and chemotherapy and anti-vascular therapy, the median PFS were 5.8, 4.4, and 5.0 months, respectively, and the median OS were 11.4, 8.2, and 8.2 months, respectively. Immunization combined with chemotherapy as first-line (22 cases) and second-line (20 cases) treatment resulted in a median PFS of 9.0 and 4.7 months, respectively (P=0.003) and a median OS of no reach and 7.8 months, respectively (P=0.007). Patients with PD-L1 expression (CPS≥1% vs. <1%) had ORRs of 37.1% and 13.3%, respectively; DCRs of 65.7% and 46.7%, respectively; median PFS of 5.8 and 4.7 months, respectively, and median OS of 11.3 and 9.3 months, respectively. Multivariate analysis showed that age, ECOG score, number of metastases, peritoneal metastasis, and number of immunotherapy lines were independent influencing factors of OS (P<0.05), and patient age was a protective factor (HR=0.498, 95%CI:0.255–0.974). Conclusions Early treatment of advanced G/GEJ adenocarcinoma with anti-PD-1 combination therapy can effectively prolong PFS and OS. Anti-PD-1 combined with chemotherapy and anti-vascular therapy have certain advantages but warrant further exploration and research. -
Key words:
- gastric cancer /
- immunotherapy /
- efficacy /
- prognosis
-
表 1 晚期G/GEJ腺癌临床基本特征 (n=118)
临床特征 例数 百分比(%) 性别 男 90 76.3 女 28 23.7 年龄(岁) ≤40 12 10.2 >40 106 89.8 ECOG评分(分) 0~1 89 75.4 ≥2 29 24.6 转移灶数(个) ≤2 50 42.4 >2 68 57.6 肝转移 是 50 42.4 否 68 57.6 腹膜转移 是 33 28.0 否 85 72.0 治疗线数 1线 27 22.9 2线 44 37.3 ≥3线 47 39.8 PD-L1表达(%) ≥1 35 29.7 <1 15 12.7 未检测 68 57.6 MMR表达 MSI 4 3.4 MSS 56 47.5 未检测 58 49.1 EBER表达 阳性 3 2.5 阴性 27 22.9 未检测 88 74.6 表 2 晚期G/GEJ腺癌中位OS单因素及多因素分析 (n=118)
临床特征 单因素 多因素 χ² P HR(95%CI) P 性别 3.217 0.073 年龄 6.741 0.009 0.498(0.255~0.974) 0.042 ECOG评分 23.780 <0.001 2.424(1.453~4.042) 0.001 转移灶数目 7.272 0.007 1.854(1.149~2.993) 0.011 肝转移 1.704 0.192 腹膜转移 14.011 <0.001 1.748(1.081~2.827) 0.023 一线 0.014 二线 vs.一线 8.670 0.003 2.345(1.146~4.800) 0.020 三线及以上 vs.一线 15.104 <0.001 2.906(1.421~5.943) 0.003 表 3 治疗相关不良反应情况 (例)
不良反应 信迪利单抗组 卡瑞利珠单抗组 特瑞普利单抗组 替雷利珠单抗组 帕博利珠单抗组 纳武利尤单抗组 (n=39) (n=48) (n=15) (n=4) (n=9) (n=3) 中性粒细胞减少 1~2级 7 15 3 1 0 0 3~4级 1 3 1 0 1 0 淋巴细胞减少 1~2级 17 26 8 1 5 2 3~4级 2 5 0 1 1 1 血红蛋白减少 1~2级 11 23 5 1 4 2 3~4级 5 10 2 0 1 0 消化道反应 1~2级 18 32 9 3 6 2 3~4级 0 0 0 0 0 0 胆红素增高 1~2级 3 2 0 1 1 0 3~4级 1 2 0 0 0 1 转氨酶增高 1~2级 3 8 1 1 3 0 3~4级 3 3 1 0 1 1 甲状腺功能减退 1~2级 4 11 3 1 0 0 3~4级 0 0 0 0 0 0 肺炎 1~2级 0 4 0 0 0 0 3~4级 0 0 0 0 1 0 蛋白尿 1~2级 3 3 1 0 0 0 3~4级 0 1 0 0 0 0 -
[1] Sung H, Ferlay J, Siegel R L, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3):209-249. doi: 10.3322/caac.21660 [2] Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015[J]. CA Cancer J Clin, 2016, 66(2):115-132. doi: 10.3322/caac.21338 [3] Qing Y, Li Q, Ren T, et al. Upregulation of PD-L1 and APE1 is associated with tumorigenesis and poor prognosis of gastric cancer[J]. Drug Des Devel Ther, 2015, 9:901-909. [4] Wu C, Zhu Y, Jiang J, et al. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance[J]. Acta Histochem, 2006, 108(1):19-24. doi: 10.1016/j.acthis.2006.01.003 [5] Janjigian Y Y, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial[J]. Lancet, 2021, 398(10294):27-40. doi: 10.1016/S0140-6736(21)00797-2 [6] Shitara K, Van Cutsem E, Bang Y J, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: The KEYNOTE-062 phase 3 randomized clinical trial[J]. JAMA Oncol, 2020, 6(10):1571-1580. doi: 10.1001/jamaoncol.2020.3370 [7] Muro K, Van Cutsem E, Narita Y, et al. Pan-Asian adapted ESMO clinical practice guidelines for the management of patients with metastatic gastric cancer: a JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS[J]. Ann Oncol, 2019, 30(1):19-33. doi: 10.1093/annonc/mdy502 [8] Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial[J]. Lancet, 2010, 376(9742):687-697. doi: 10.1016/S0140-6736(10)61121-X [9] Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial[J]. Lancet Oncol, 2014, 15(11):1224-1235. doi: 10.1016/S1470-2045(14)70420-6 [10] Shitara K, Ozguroglu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial[J]. Lancet, 2018, 392(10142):123-133. doi: 10.1016/S0140-6736(18)31257-1 [11] Xu J, Zhang Y, Jia R, et al. Anti-PD-1 antibody SHR-1210 combined with apatinib for advanced hepatocellular carcinoma, gastric, or esophagogastric junction cancer: an open-label, dose escalation and expansion study[J]. Clin Cancer Res, 2019, 25(2):515-523. doi: 10.1158/1078-0432.CCR-18-2484 [12] Song Y, Li N, Li Q, et al. HX008, an anti-PD1 antibody, plus irinotecan as second-line treatment for advanced gastric or gastroesophageal junction cancer: a multicenter, single-arm phase Ⅱ trial[J]. J Immunother Cancer, 2020, 8(2):e001279. [13] Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial[J]. Lancet, 2017, 390(10111):2461-2471. [14] Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial[J]. JAMA Oncol, 2018, 4(5):e180013. doi: 10.1001/jamaoncol.2018.0013 [15] Yi M, Jiao D, Qin S, et al. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment[J]. Mol Cancer, 2019, 18(1):60. doi: 10.1186/s12943-019-0974-6 [16] Heinhuis KM, Ros W, Kok M, et al. Enhancing antitumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors[J]. Ann Oncol, 2019, 30(2):219-235. doi: 10.1093/annonc/mdy551 [17] Bang YJ, Kang YK, Catenacci DV, et al. Pembrolizumab alone or in combination with chemotherapy as first-line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: results from the phase Ⅱ nonrandomized KEYNOTE-059 study[J]. Gastric Cancer, 2019, 22(4):828-837. doi: 10.1007/s10120-018-00909-5 [18] Boku N, Ryu MH, Kato K, et al. Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, phaseⅡ trial (ATTRACTION-4)[J]. Ann Oncol, 2019, 30(2):250-258. doi: 10.1093/annonc/mdy540 [19] Fukuoka S, Hara H, Takahashi N, et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase Ⅰb trial (REGONIVO, EPOC1603)[J]. J Clin Oncol, 2020, 38(18):2053-2061. doi: 10.1200/JCO.19.03296 [20] Kawazoe A, Fukuoka S, Nakamura Y, et al. Lenvatinib plus pembrolizumab in patients with advanced gastric cancer in the first-line or second-line setting (EPOC1706): an open-label, single-arm, phase 2 trial[J]. Lancet Oncol, 2020, 21(8):1057-1065. doi: 10.1016/S1470-2045(20)30271-0 [21] Janjigian YY, Bendell J, Calvo E, et al. CheckMate-032 study: efficacy and safety of nivolumab and nivolumab plus ipilimumab in patients with metastatic esophagogastric cancer[J]. J Clin Oncol, 2018, 36(28):2836-2844. doi: 10.1200/JCO.2017.76.6212 [22] Liu X, Yang Z, Latchoumanin O, et al. Antagonizing programmed death-1 and programmed death ligand-1 as a therapeutic approach for gastric cancer[J]. Therap Adv Gastroenterol, 2016, 9(6):853-860. doi: 10.1177/1756283X16658251 [23] Kim JW, Nam KH, Ahn SH, et al. Prognostic implications of immunosuppressive protein expression in tumors as well as immune cell infiltration within the tumor microenvironment in gastric cancer[J]. Gastric Cancer, 2016, 19(1):42-52. doi: 10.1007/s10120-014-0440-5 [24] Gu L, Chen M, Guo D, et al. PD-L1 and gastric cancer prognosis: A systematic review and meta-analysis[J]. PLoS One, 2017, 12(8):e182692. [25] Ma C, Patel K, Singhi AD, et al. Programmed Death-Ligand 1 Expression Is Common in Gastric Cancer Associated With Epstein-Barr Virus or Microsatellite Instability[J]. Am J Surg Pathol, 2016, 40(11):1496-1506. [26] Chao J, Fuchs CS, Shitara K, et al. Assessment of pembrolizumab therapy for the treatment of microsatellite instability-high gastric or gastroesophageal junction cancer among patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 clinical trials[J]. JAMA Oncol, 2021, 7(6):895-902. doi: 10.1001/jamaoncol.2021.0275 [27] Kim ST, Cristescu R, Bass AJ, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer[J]. Nat Med, 2018, 24(9):1449-1458. doi: 10.1038/s41591-018-0101-z -



 下载:
下载: