Research progress on chimeric antigen receptor T cells in gastric cancer immunotherapy
-
摘要: 胃癌是最常见的恶性肿瘤之一,部分病例由于诊断较晚和治疗不佳,患者的整体生存期较差。近几年,嵌合抗原受体修饰T细胞(chimeric antigen receptor T cells,CAR-T)疗法在肿瘤治疗研究领域受到相当大的关注,且已在血液系统肿瘤中发挥了重要作用,但在实体肿瘤因受到靶点特异性,肿瘤微环境等因素影响而作用受限。尽管如此,各种临床前和临床实验已证实CAR-T细胞免疫治疗是胃癌一种有前景的治疗方法,并具有有别于其他实体瘤的优势,但是仍面临诸多挑战。本文将系统介绍CAR-T疗法在胃癌中的最新研究进展。Abstract: Gastric cancer is one of the most common malignant tumors. Due to late diagnosis and less effective treatment in some cases, the overall survival rate is poor. In recent years, chimeric antigen receptor T cell (CAR-T cell) therapy has received considerable attention in the field of cancer treatment and has played an important role in the treatment of hematological tumors. However, the theraputic efficacy in solid tumors are affected by target specificity, the tumor microenvironment, and other factors. Various preclinical and clinical trials have demonstrated that CAR-T cell immunotherapy is a promising treatment approach for gastric cancer. It exhibits different advantages when treating other solid tumors but still faces many challenges in development. In this review, the latest research progress in CAR-T therapy in gastric cancer will be examined.
-
Key words:
- gastric cancer /
- CAR-T therapy /
- biological basis /
- clinical application
-
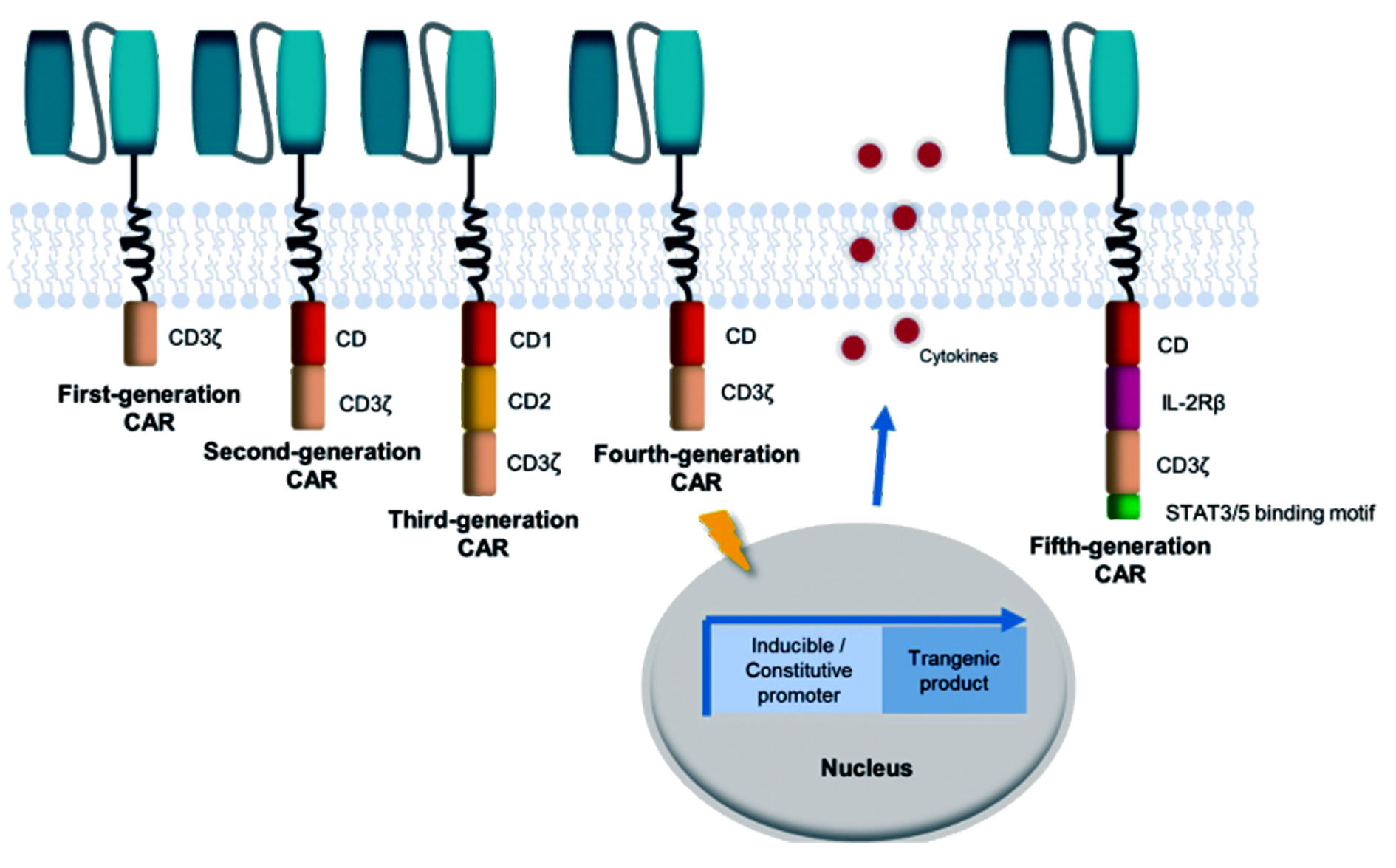
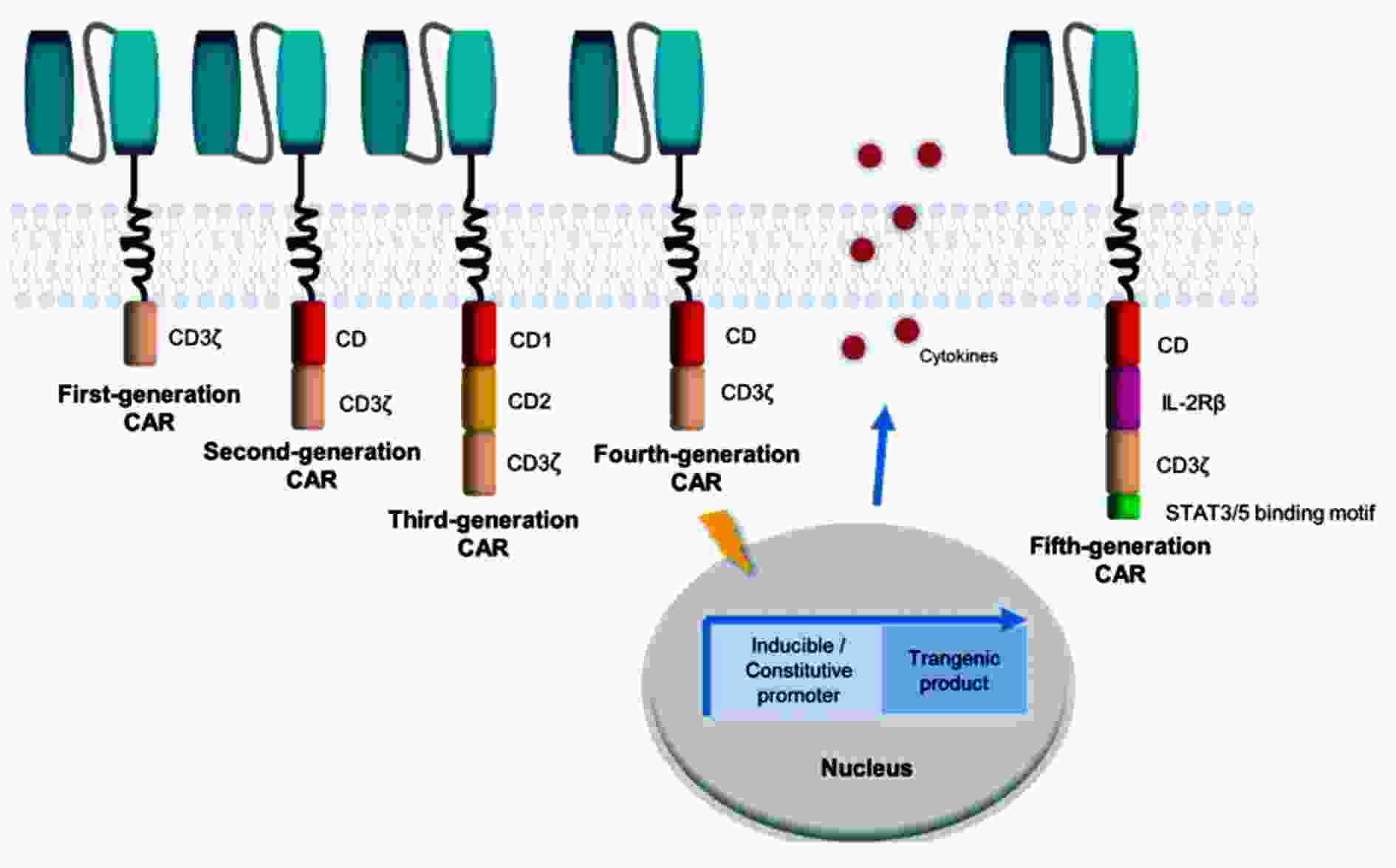
图 1 CAR的结构和演化的示意图[3]
表 1 美国FDA批准CAR-T治疗药物
kymriah yescarta tecartus breyanzi abecma 批准时间 2017.8.30 2017.10.18 2020.7.24 2021.2.5 2021.3.26 靶点 CD19 CD19 CD19 CD19 BCMA 公司 诺华 吉利德 吉利德 百时美施贵宝 百时美施贵宝/蓝鸟生物 适应征 25岁以下复发或难治性B系急性淋巴细胞白血病(B-ALL) 成人复发或难治性大B细胞淋巴瘤 成人复发或难治性套细胞淋巴瘤 成人复发或难治性大B细胞淋巴瘤 成人复发或难治性多发性骨髓瘤 成人复发或难治性大B细胞淋巴瘤 -
[1] Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries[J]. CA Cancer J Clin, 2021, 71(3):209-249. doi: 10.3322/caac.21660 [2] Siddiqui RS, Sardar M. A Systematic Review of the Role of Chimeric Antigen Receptor T (CAR-T) Cell Therapy in the Treatment of Solid Tumors[J]. Cureus, 2021, 13(4):e14494. [3] Abreu TR, Fonseca NA, Gonçalves N, et al. Current challenges and emerging opportunities of CAR-T cell therapies[J]. J Control Release, 2020, 319:246-261. [4] Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity[J]. ProcNatl Acad Sci U S A, 1989, 86(24):10024-10028. doi: 10.1073/pnas.86.24.10024 [5] Ramello MC, Benzaïd I, Kuenzi BM, et al. An immunoproteomic approach to characterize the CAR interactome and signalosome[J]. SciSignal, 2019, 12(568):eaap9777. [6] Fraietta JA, Lacey SF, Orlando EJ, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia[J]. NatMed, 2018, 24(5):563-571. [7] 李成功,梅恒,胡豫.流式细胞术在CAR-T细胞治疗中的应用进展[J].中国实验血液学杂志,2020,28(1):329-332. [8] Salem DA, Maric I, Yuan CM, et al. Quantification of B-cell maturation antigen, a target for novel chimeric antigen receptor T-cell therapy in Myeloma[J]. Leuk Res, 2018, 71:106-111. [9] Cushman-Vokoun AM, Voelkerding KV, Fung MK, et al. A Primer on Chimeric Antigen Receptor T-cell Therapy: What Does It Mean for Pathologists?[J]. Arch Pathol Lab Med, 2021, 145(6):704-716. [10] Han YL, Liu CY, Li GH, et al. Antitumor effects and persistence of a novel HER2 CAR T cells directed to gastric cancer in preclinical models[J]. AmJ Cancer Res, 2018, 8(1):106-119. [11] Song YJ, Tong C, Wang Y, et al. Effective and persistent antitumor activity of HER2-directed CAR-T cells against gastric cancer cells in vitro and xenotransplanted tumors in vivo[J]. Protein Cell, 2018, 9(10):867-878. doi: 10.1007/s13238-017-0384-8 [12] Luo FF, Qian JW, Yang J, et al. Bifunctional αHER2/CD3 RNA-engineered CART-like human T cells specifically eliminate HER2(+) gastric cancer[J]. Cell Res, 2016, 26(7):850-853. doi: 10.1038/cr.2016.81 [13] Wang LN, Ma N, Okamoto S, et al. Efficient tumor regression by adoptively transferred CEA-specific CAR-T cells associated with symptoms of mild cytokine release syndrome[J]. Oncoimmunology, 2016, 5(9):e1211218. doi: 10.1080/2162402X.2016.1211218 [14] Shibaguchi H, Luo NX, Shirasu N, et al. Enhancement of antitumor activity by using a fully human gene encoding a single-chain fragmented antibody specific for carcinoembryonic antigen[J]. Onco Targets Ther, 2017, 10:3979-3990. [15] Chi XW, Yang PW, Zhang EH, et al. Significantly increased anti-tumor activity of carcinoembryonic antigen-specific chimeric antigen receptor T cells in combination with recombinant human IL-12[J]. Cancer Med, 2019, 8(10):4753-4765. doi: 10.1002/cam4.2361 [16] Jiang H, Shi ZM, Wang P, et al. Claudin18.2-Specific Chimeric Antigen Receptor Engineered T Cells for the Treatment of Gastric Cancer[J]. JNatl Cancer Inst, 2019, 111(4):409-418. doi: 10.1093/jnci/djy134 [17] Zhan XB, Wang B, Li ZH, et al. Phase I trial of Claudin 18.2-specific chimeric antigen receptor T cells for advanced gastric and pancreatic adenocarcinoma[J]. 2019, 37(15_suppl): 2509-2509. [18] Jing X, Liang HP, Hao CH, et al. Overexpression of MUC1 predicts poor prognosis in patients with breast cancer[J]. Oncol Rep, 2019, 41(2):801-810. [19] Wilkie S, Picco G, Foster J, et al. Retargeting of human T cells to tumor-associated MUC1: the evolution of a chimeric antigen receptor[J]. JImmunol, 2008, 180(7):4901-4909. doi: 10.4049/jimmunol.180.7.4901 [20] Lv J, Zhao RC, Wu D, et al. Mesothelin is a target of chimeric antigen receptor T cells for treating gastric cancer[J]. J Hematol Oncol, 2019, 12(1):18. [21] Zhang ZW, Jiang DQ, Yang H, et al. Modified CAR T cells targeting membrane-proximal epitope of mesothelin enhances the antitumor function against large solid tumor[J]. Cell Death Dis, 2019, 10(7):476. doi: 10.1038/s41419-019-1711-1 [22] Kim M, Pyo S, Kang CH, et al. Folate receptor 1 (FOLR1) targeted chimeric antigen receptor (CAR) T cells for the treatment of gastric cancer[J]. PloS One, 2018, 13(6):e0198347. doi: 10.1371/journal.pone.0198347 [23] Liu XQ, Sun ML, Yu S, et al. Potential therapeutic strategy for gastric cancer peritoneal metastasis by NKG2D ligands-specific T cells[J]. Onco Targets Ther, 2015, 8:3095-3104. [24] Tao KL, He M, Tao F, et al. Development of NKG2D-based chimeric antigen receptor-T cells for gastric cancer treatment[J]. Cancer Chemother Pharmacol, 2018, 82(5):815-827. [25] Zhao W, Zhu HJ, Zhang S, et al. Trop2 is overexpressed in gastric cancer and predicts poor prognosis[J]. Oncotarget, 2016, 7(5):6136-6145. [26] Zhao W, Jia LZ, Zhang MJ, et al. The killing effect of novel bi-specific Trop2/PD-L1 CAR-T cell targeted gastric cancer[J]. Am J Cancer Res, 2019, 9(8):1846-1856. [27] Yuan J. Abstract 3766: MG7-car, a first-in-class T-cell therapy for gastric cancer[J]. Cancer Res, 2017, 77(13_Suppl): 3766-3766. [28] Lü P, Qiu SL, Pan Y, et al. Preclinical Chimeric Antibody Chimeric Antigen Receptor T Cell Progress in Digestive System Cancers[J]. Cancer Biother Radiopharm, 2021, 36(4):307-315. [29] 曹玉环,苑学礼.CAR-T细胞在实体肿瘤治疗中的应用进展[J].医学综述,2020,26(23):4634-4642. [30] Suarez ER, Chang DK, Sun JS, et al. Chimeric antigen receptor T cells secreting anti-PD-L1 antibodies more effectively regress renal cell carcinoma in a humanized mouse model[J]. Oncotarget, 2016, 7(23):34341-34355. doi: 10.18632/oncotarget.9114 [31] Chen C, Li KS, Jiang H, et al. Development of T cells carrying two complementary chimeric antigen receptors against glypican-3 and asialoglycoprotein receptor 1 for the treatment of hepatocellular carcinoma[J]. Cancer ImmunolImmunother, 2017, 66(4):475-489. doi: 10.1007/s00262-016-1949-8 [32] Kosti P, Opzoomer JW, Larios-Martinez KI, et al. Hypoxia-sensing CAR T cells provide safety and efficacy in treating solid tumors[J]. Cell Rep Med, 2021, 2(4):100227. doi: 10.1016/j.xcrm.2021.100227 [33] Kershaw MH, Westwood JA, Parker LL, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer[J]. ClinCancer Res, 2006, 12(20 Pt 1): 6106-6115. [34] Guedan S, Posey AD, Shaw C, et al. Enhancing CAR T cell persistence through ICOS and 4-1BB costimulation[J]. JCI Insight, 2018, 3(1):e96976. doi: 10.1172/jci.insight.96976 [35] Sofi MH, Wu YX, Schutt SD, et al. Thioredoxin-1 confines T cell alloresponse and pathogenicity in graft-versus-host disease[J]. J Clin Invest, 2019, 129(7):2760-2774. -



 下载:
下载: