Anlotinib monotherapy for refractory metastatic colorectal cancer: a single-center data analysis of a multicenter phase Ⅲ trial
-
摘要:
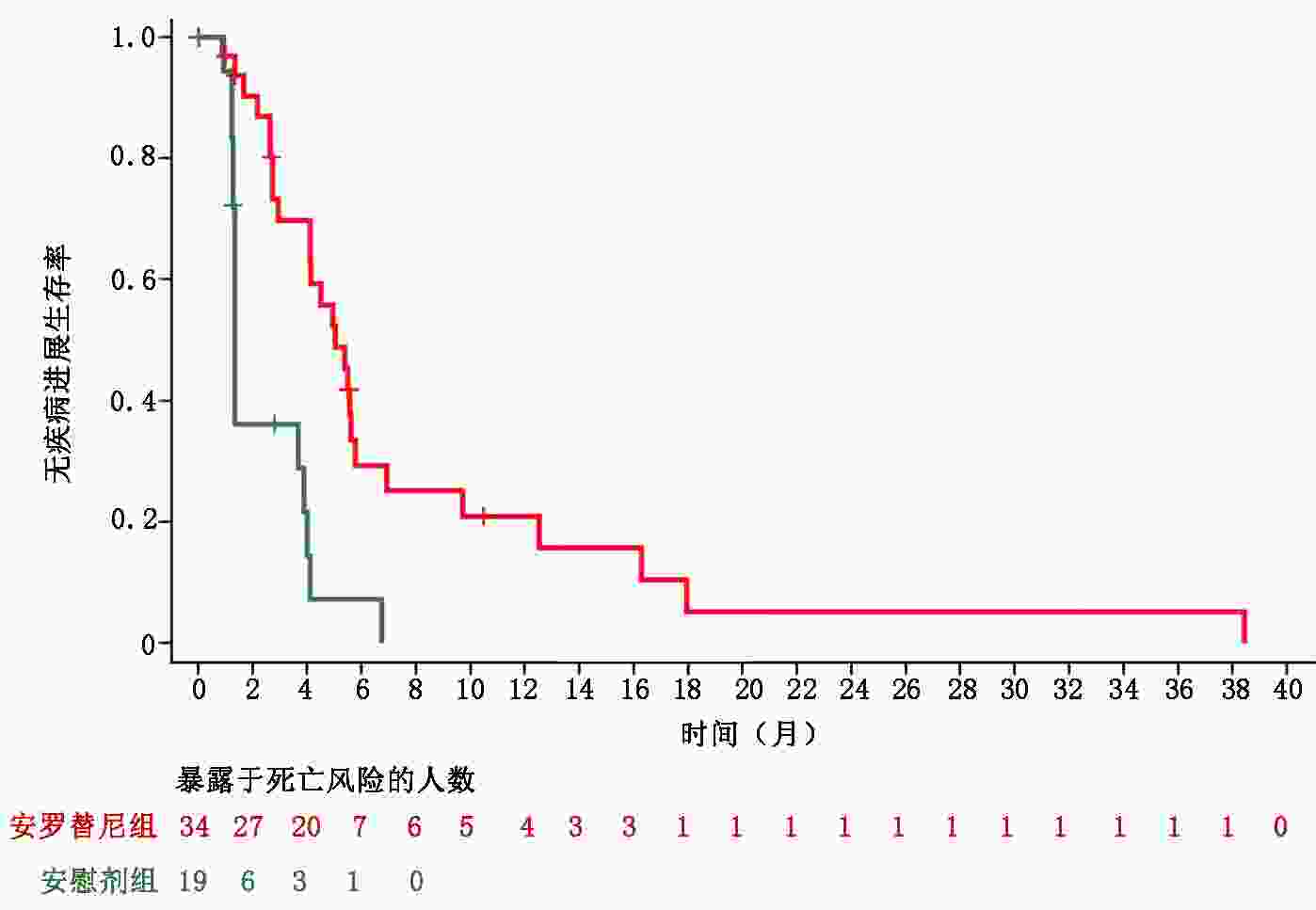
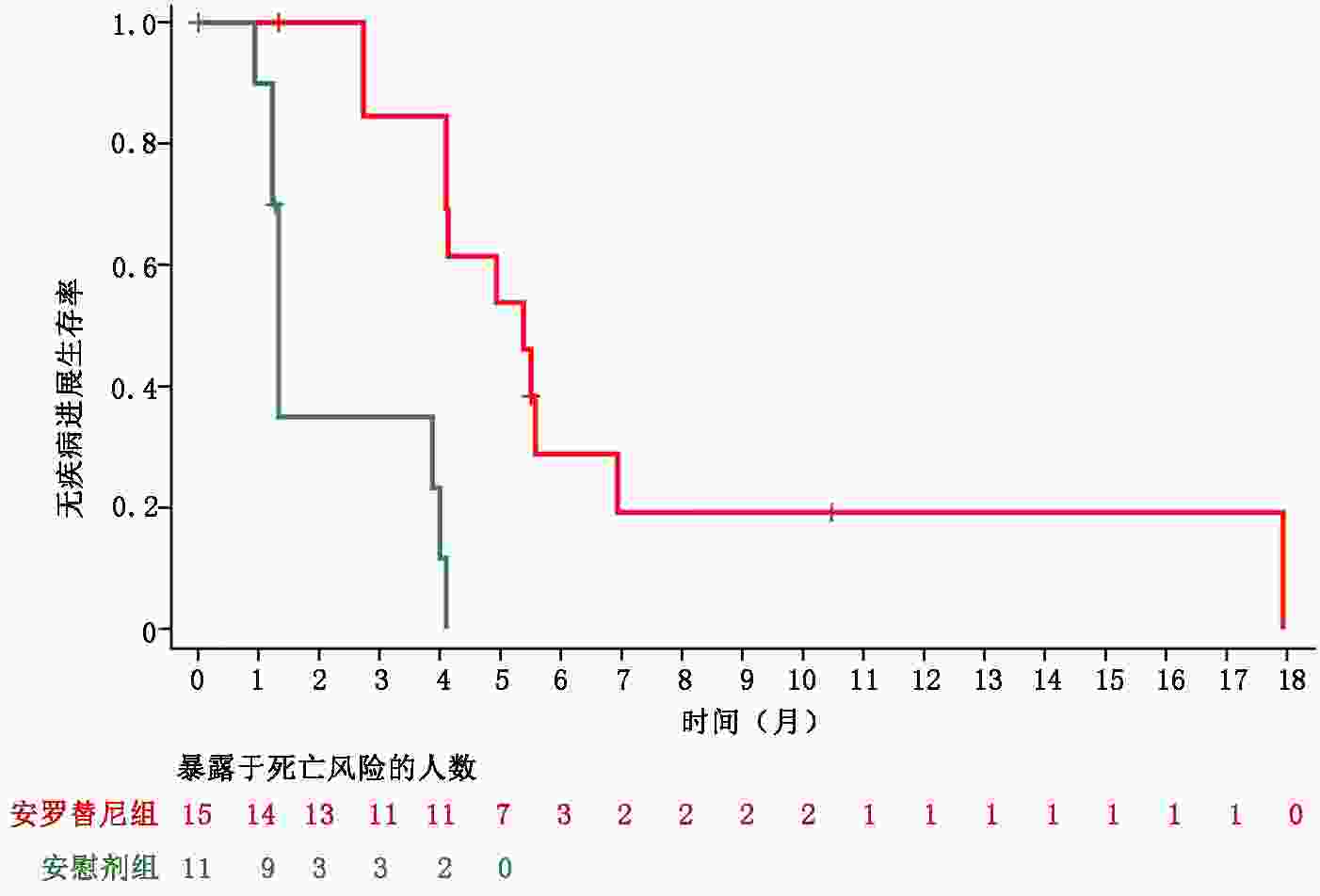
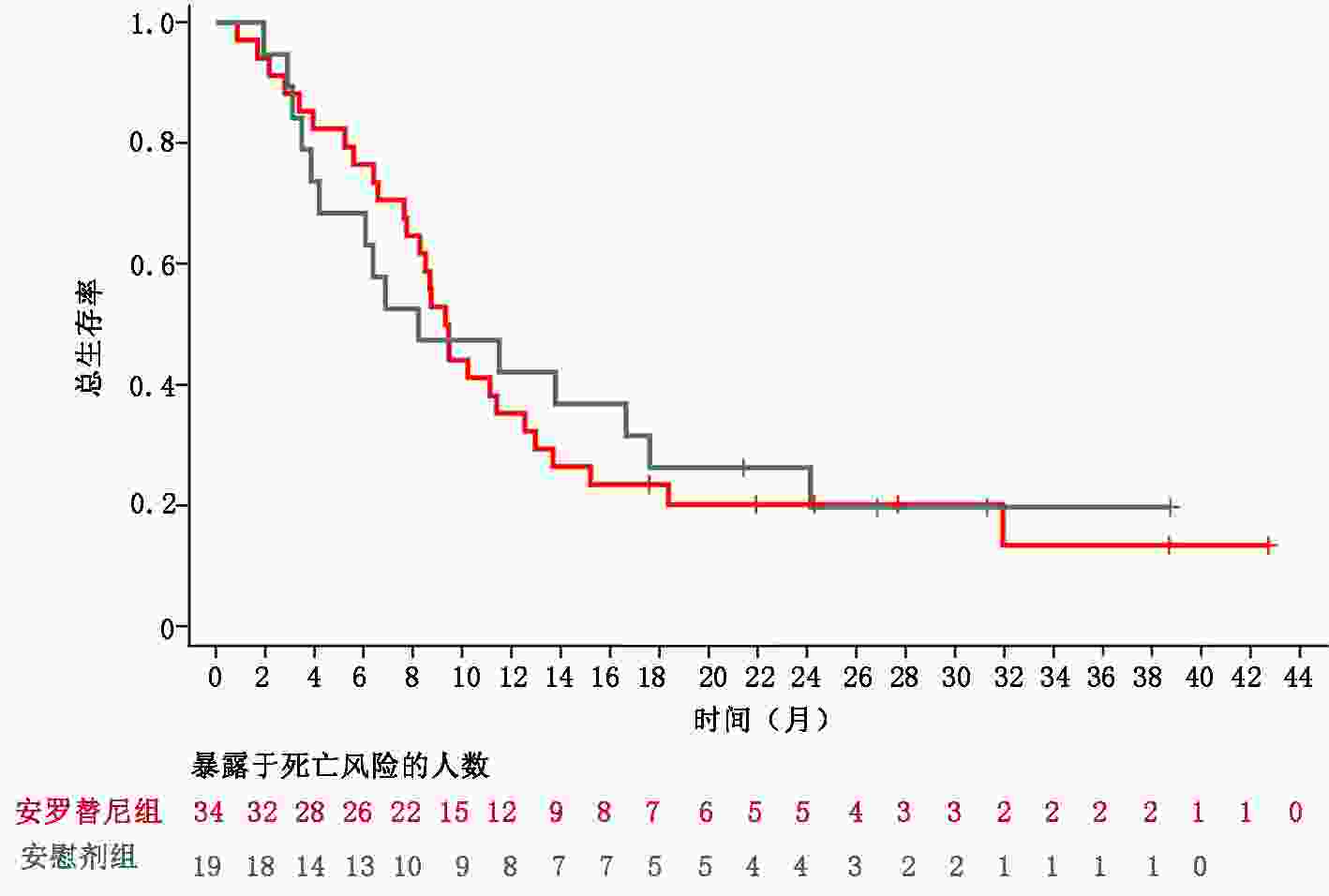
目的 分析安罗替尼三线治疗难治型晚期结直肠癌的疗效及安全性。 方法 收集并分析2014年9月至2016年8月中国医学科学院肿瘤医院收治的53例晚期结直肠癌患者临床信息,观察、评估安罗替尼的疗效及安全性。 结果 患者随机分为安罗替尼组和安慰剂组。安罗替尼组与安慰剂组的中位总生存期(overall survival,OS)分别为9.37个月和8.23个月;中位无进展生存期(progression-free survival,PFS)分别为5.03个月和1.33个月(HR=0.27,95%CI:0.13~0.54)。两组患者客观缓解率(objective response rate,ORR)分别为2.94%和0;安罗替尼组的疾病控制率(disease control rate,DCR)显著高于安慰剂组(88.24% vs. 36.84%,P<0.001)。26例RAS/BRAF野生型患者中,安罗替尼组中位PFS显著长于安慰剂组(5.37个月 vs. 1.33个月),DCR显著优于安慰剂组(93.33% vs. 36.36%,P=0.003)。最常见的≥3级安罗替尼相关不良事件为高血压(17.65%)、腹泻(8.82%)和掌跖红肿综合征(8.82%)。 结论 安罗替尼后线治疗晚期结直肠癌可改善患者无进展生存期(progression-free survival,PFS),提升DCR,且安全性良好,为晚期结直肠癌的治疗提供了新思路。然而对于RAS/BRAF野生型人群的临床获益趋势有待进一步验证。 Abstract:Objective To assess the efficacy and safety of anlotinib for patients with metastatic colorectal cancer (mCRC) who had received at least two lines of standard therapy. Methods The clinical data of 53 patients with refractory colorectal cancer treated with anlotinib at National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital from September 2014 to August 2016 were collected and analyzed. The efficacy and safety of anlotinib were observed and evaluated. Results Patients were randomly assigned into anlotinib group and placebo group. The median overall survival (OS) times for the anlotiniband groups and the placebo groups were 8.2 months and 9.3 months, respectively. The median progression-free survival (PFS) measures for the anlotinib groups and the placebo groups were 5.03 months and 1.33 months, respectively (hazard ratio, HR=0.27, 95% confidence interval, 95%CI: 0.13-0.54). The objective response rates (ORR) for the two groups were 2.94% and 0, respectively. The disease control rate (DCR) was better in the anlotinib group than in the placebo group (88.24% vs. 36.84%, P<0.00 1). In the 26 patients with RAS/BRAF wild type, the median PFS significantly improved in the anlotinib group compared with that of the placebo group (5.37 months vs. 1.33 months). The DCR of anlotinib was also higher than that of the placebo (93.33% vs. 36.36%, P=0.003). The most common above grade 3 anlotinib-related adverse events were hypertension (17.65%), diarrhea (8.82%), and hand-foot skin reaction (8.82%). Conclusions Anlotinib is effective as a third- or later-line therapy for mCRC, with significant PFS benefits and increased DCR with acceptable toxicity. Therefore, it maybe an option for treating mCRC. Potential benefits for patients with RAS/BRAF wild-type cancer should be confirmed with further clinical trials. -
Key words:
- metastatic colorectal cancer (mCRC) /
- anlotinib /
- efficacy /
- survival analysis /
- safety
-
表 1 患者基线情况
项目 安罗替尼
n=34安慰剂
n=19P 年龄 (岁) 1.000 <65 30(88.24) 17(89.47) ≥65 4(11.76) 2(10.53) 性别 0.920 男性 21(61.76) 12(63.16) 女性 13(38.24) 7(36.84) ECOG PS评分(分) 0.194 0 17(50.00) 6(31.58) 1 17(50.00) 13(68.42) 疾病原发部位 0.489 结肠 20(58.82) 8(42.11) 结直肠 3(8.82) 2(10.53) 直肠 11(32.35) 9(47.37) 左右结肠部位 1.000 右 4(11.76) 2(10.53) 左 29(85.29) 17(89.47) 不详 1(2.94) 0(0) 抗血管靶向药治疗史 0.990 否 25(73.53) 14(73.68) 是 9(26.47) 5(26.32) 初诊至转移时间(月) 0.234 ≥18 3(8.82) 4(21.05) <18 31(91.18) 15(78.95) KRAS状态 1.000 突变型 12(35.29) 6(31.58) 野生型 18(52.94) 11(57.89) 不详 4(11.76) 2(10.53) RAS(KRAS/NRAS/BRAF)状态 0.615 突变型 15(44.12) 6(31.58) 野生型 15(44.12) 11(57.89) 不详 4(11.76) 2(10.53) 结直肠癌手术史 1.000 否 6(17.65) 4(21.05) 是 28(82.35) 15(78.95) 肝转移 0.869 否 10(29.41) 6(31.58) 是 24(70.59) 13(68.42) 放疗史 1.000 否 26(76.47) 14(73.68) 是 8(23.53) 5(26.32) ()内单位为% 表 2 RAS/BRAF野生型患者的OS及PFS分析
生存分析 例数 删失 中位值(95%CI) P HR(95%CI) OS 安慰剂 11 3 11.50(3.87, NE) 安罗替尼 15 3 11.10(6.40, 12.97) 0.810 1.12(0.45, 2.76) PFS 安慰剂 11 2 1.33(0.93, 4.00) 安罗替尼 15 4 5.37(4.10, 6.93) <0.001 0.11(0.03, 0.38) 表 3 全部患者的ORR及DCR分析
最佳客观疗效 安罗替尼
(n=34)安慰剂
(n=19)P PR n(%) 1(2.94) 0(0) SD n(%) 29(85.29) 7(36.84) PD n(%) 1(2.94) 11(57.89) NA n(%) 3(8.82) 1(5.26) ORR %(95%CI) 2.94(0.07, 15.33) − 1.000 DCR %(95%CI) 88.24(72.55, 96.70) 36.84(16.29, 61.64) <0.001 表 4 RAS/BRAF野生型患者ORR及DCR分析
最佳客观疗效 安罗替尼(n=15) 安慰剂(n=11) P PR n(%) 1(6.67) 0(0) SD n(%) 13(86.67) 4(36.36) PD n(%) 0(0) 6(54.55) NA n(%) 1(6.67) 1(9.09) ORR %(95%CI) 6.67(0.17, 31.95) − 1.000 DCR %(95%CI) 93.33(68.05, 99.83) 36.36(10.93, 69.21) 0.003 表 5 全部患者安全性分析(任一组别发生率≥5%的不良反应)n(%)
不良反应 安罗替尼(n=34) 安慰剂(n=19) 总计 ≥3级 总计 ≥3级 疲乏 23(67.65) 2(5.88) 9(47.37) 0(0) 甲状腺功能减退 22(64.71) 0(0) 0(0) 0(0) 高血压 21(61.76) 6(17.65) 5(26.32) 0(0) 结合胆红素升高 20(58.82) 2(5.88) 5(26.32) 0(0) 蛋白尿 17(50.00) 1(2.94) 3(15.79) 0(0) 腹泻 17(50.00) 3(8.82) 4(21.05) 0(0) 低密度脂蛋白升高 15(44.12) 0(0) 7(36.84) 0(0) 掌跖红肿综合征 15(44.12) 3(8.82) 1(5.26) 0(0) 高甘油三酯血症 14(41.18) 0(0) 2(10.53) 0(0) 食欲下降 13(38.24) 0(0) 1(5.26) 0(0) 丙氨酸氨基转移酶升高 12(35.29) 0(0) 4(21.05) 0(0) 高胆固醇血症 12(35.29) 0(0) 6(31.58) 0(0) 天门冬氨酸氨基转移酶升高 12(35.29) 0(0) 5(26.32) 0(0) 尿红细胞阳性 11(32.35) 0(0) 1(5.26) 0(0) 潜血阳性 11(32.35) 0(0) 1(5.26) 0(0) 血胆红素升高 11(32.35) 0(0) 2(10.53) 0(0) 腹痛 10(29.41) 0(0) 4(21.05) 0(0) 中性粒细胞计数降低 8(23.53) 0(0) 0(0) 0(0) 白细胞计数降低 6(17.65) 0(0) 0(0) 0(0) 发音困难 6(17.65) 0(0) 0(0) 0(0) 口咽疼痛 6(17.65) 0(0) 1(5.26) 0(0) 口腔溃疡 5(14.71) 1(2.94) 0(0) 0(0) 血非结合胆红素升高 5(14.71) 0(0) 2(10.53) 0(0) 脂肪酶升高 5(14.71) 1(2.94) 0(0) 0(0) 头痛 4(11.76) 0(0) 1(5.26) 0(0) 心电图 QT 间期延长 4(11.76) 1(2.94) 1(5.26) 0(0) 血小板计数降低 4(11.76) 0(0) 2(10.53) 0(0) 鼻衄 3(8.82) 0(0) 0(0) 0(0) 恶心 3(8.82) 0(0) 2(10.53) 0(0) 气胸 3(8.82) 0(0) 0(0) 0(0) 脱发 3(8.82) 0(0) 0(0) 0(0) 心肌缺血 3(8.82) 0(0) 0(0) 0(0) 牙疼 3(8.82) 0(0) 1(5.26) 0(0) 背痛 2(5.88) 0(0) 1(5.26) 0(0) 齿龈疼痛 2(5.88) 0(0) 0(0) 0(0) 淀粉酶升高 2(5.88) 0(0) 1(5.26) 0(0) 耳鸣 2(5.88) 0(0) 0(0) 0(0) 肌痛 2(5.88) 0(0) 2(10.53) 0(0) 皮疹 2(5.88) 0(0) 1(5.26) 0(0) 上腹痛 2(5.88) 0(0) 0(0) 0(0) 室性期外收缩 2(5.88) 0(0) 1(5.26) 0(0) 腹胀 1(2.94) 0(0) 1(5.26) 0(0) 呼吸困难 1(2.94) 0(0) 1(5.26) 0(0) 呕吐 1(2.94) 0(0) 1(5.26) 0(0) 胃食管反流病 0(0) 0(0) 1(5.26) 0(0) -
[1] Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3):209-249. doi: 10.3322/caac.21660 [2] Zhang S, Sun K, Zheng R, et al. Cancer incidence and mortality in China, 2015[J]. J Nat Cancer Center, 2021, 1(1):2-11. doi: 10.1016/j.jncc.2020.12.001 [3] Van Cutsem E, Cervantes A, Nordlinger B, et al. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up[J]. Ann Oncol, 2014, 25 (Suppl 3):iii1-9. [4] Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019[J]. CA Cancer J Clin, 2019, 69(5):363-385. doi: 10.3322/caac.21565 [5] 中华人民共和国国家卫生健康委员会.中国结直肠癌诊疗规范(2020年版)[J].中华外科杂志,2020,8(58):561-585. [6] Han B, Li K, Wang Q, et al. Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: The ALTER 0303 phase 3 randomized clinical trial[J]. JAMA Oncol, 2018, 4(11):1569-1575. doi: 10.1001/jamaoncol.2018.3039 [7] Chi Y, Fang Z, Hong X, et al. Safety and efficacy of anlotinib, a multikinase angiogenesis inhibitor, in patients with refractory metastatic soft-tissue sarcoma[J]. Clin Cancer Res, 2018, 24(21):5233-5238. doi: 10.1158/1078-0432.CCR-17-3766 [8] Sun Y, Du F, Gao M, et al. Anlotinib for the treatment of patients with locally advanced or metastatic medullary thyroid cancer[J]. Thyroid, 2018, 28(11):1455-1461. doi: 10.1089/thy.2018.0022 [9] Ruan X, Shi X, Dong Q, et al. Antitumor effects of anlotinib in thyroid cancer[J]. Endocr Relat Cancer, 2019, 26(1):153-164. doi: 10.1530/ERC-17-0558 [10] Zhou AP, Bai Y, Song Y, et al. Anlotinib versus sunitinib as first-line treatment for metastatic renal cell carcinoma: A randomized phase II clinical trial[J]. Oncologist, 2019, 24(8):e702-e708. doi: 10.1634/theoncologist.2018-0839 [11] He C, Wu T, Hao Y. Anlotinib induces hepatocellular carcinoma apoptosis and inhibits proliferation via Erk and Akt pathway[J]. Biochem Biophys Res Commun, 2018, 503(4):3093-3099. doi: 10.1016/j.bbrc.2018.08.098 [12] Chi Y, Shu Y, Ba Y, et al. Anlotinib monotherapy for refractory metastatic colorectal cancer: A double-blinded, placebo-controlled, randomized phase Ⅲ trial (ALTER0703)[J]. Oncologist, 2021, 26(10):e1693-e1703. [13] Michiels S, Saad ED, Buyse M. Progression-free survival as a surrogate for overall survival in clinical trials of targeted therapy in advanced solid tumors[J]. Drugs, 2017, 77(7):713-719. doi: 10.1007/s40265-017-0728-y [14] Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase Ⅲ Trial[J]. J Clin Oncol, 2020, 38(3):193-202. doi: 10.1200/JCO.19.01307 [15] Piccart M, Hortobagyi GN, Campone M, et al. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: overall survival results from BOLERO-2[J]. Ann Oncol, 2014, 25(12):2357-2362. doi: 10.1093/annonc/mdu456 [16] Gravis G, Fizazi K, Joly F, et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomised, open-label, phase 3 trial[J]. Lancet Oncol, 2013, 14(2):149-158. doi: 10.1016/S1470-2045(12)70560-0 [17] Herbst RS, Sun Y, Eberhardt WE, et al. Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer (ZODIAC): a double-blind, randomised, phase 3 trial[J]. Lancet Oncol, 2010, 11(7):619-626. doi: 10.1016/S1470-2045(10)70132-7 [18] Li J, Qin S, Xu R, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial[J]. Lancet Oncol, 2015, 16(6):619-629. doi: 10.1016/S1470-2045(15)70156-7 [19] Li J, Qin S, Xu RH, et al. Effect of fruquintinib vs placebo on overall survival in patients with previously treated metastatic colorectal cancer: the FRESCO randomized clinical trial[J]. Jama, 2018, 319(24):2486-2496. doi: 10.1001/jama.2018.7855 [20] Rui Y, Wang C, Zhou Z, et al. K-Ras mutation and prognosis of colorectal cancer: a meta-analysis[J]. Hepatogastroenterology, 2015, 62(137):19-24. [21] 孙屏,郭兴美,吕慧,等.微卫星不稳定和RAS基因突变与Ⅲ~Ⅳ期大肠癌预后的相关性[J].临床与病理杂志,2020,40(10):12. [22] Ding KF, Liu Y, Du J, et al. Updated results of ALTER-C002: Anlotinib combined with CAPEOX in first-line treatment of patients with RAS/BRAF wild-type unresectable metastatic colorectal cancer[J]. J Clin Oncol, 2021, 39(15_Suppl):e15564-e15564. doi: 10.1200/JCO.2021.39.15_suppl.e15564 -



 下载:
下载: