Analysis of diagnosis and treatment status of pseudomyxoma peritonei based on real-world data
-
摘要:
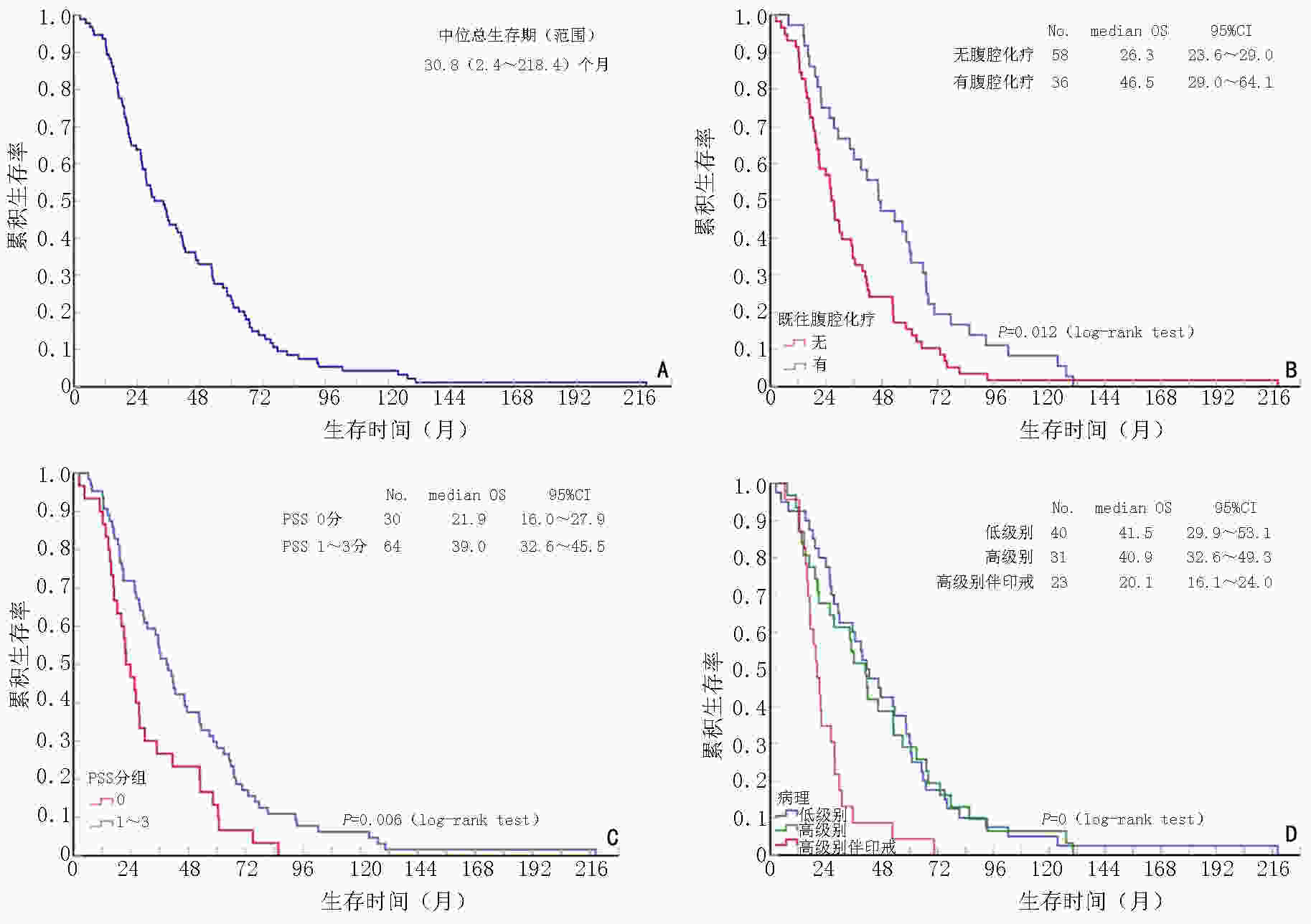
目的 基于真实世界数据分析腹膜假黏液瘤患者的诊治现状、自然病程及预后因素。 方法 回顾性分析2009年2月至2020年7月在首都医科大学附属北京世纪坛医院就诊的具有完整自然病程的腹膜假黏液瘤患者的相关资料,包括临床病理特征、非规范化治疗情况(误诊时间、误治时间、既往抗肿瘤治疗情况)、肿瘤细胞减灭术+腹腔热灌注化疗(cytoreductive surgery+hyperthermic intraperitoneal chemotherapy,CRS+HIPEC)治疗情况[手术时间、术中输血情况、腹膜癌指数(peritoneal cancer index,PCI)评分、细胞减灭程度(completeness of cytoreduction,CC)评分、脏器切除数量、腹膜切除区域数量、严重不良事件(serious adverse event,SAE)等]、随访生存时间,随访终点为患者死亡。采用Kaplan -Meier法绘制生存曲线,组间比较采用Log-rank检验。影响5年生存的预后因素采用Cox比例风险回归模型进行单因素和多因素分析。 结果 共纳入94例患者,其中男性57例(60.6%),女性37例(39.4%), 中位年龄54(24~76)岁,既往抗肿瘤治疗者59例(62.8%),中位误诊时间0.8(0~62.5)个月,中位误治时间15.3(0~214.8)个月。所有患者均行CRS+HIPEC治疗,中位手术时间10.1(4.8~16.5)h,中位脏器切除数2(0~8)个,中位腹膜切除区域数5(0~9)个,中位PCI评分32(3~39)分,CC评分2~3分者达80.9%(76/94),SAE发生率35.1%(33/94)。94例患者中位总生存期30.8(2.4~218.4)个月,1、2、3、5年生存率分别为96.8%、63.8%、44.7%和23.4%。分层分析显示,既往腹腔化疗(46.5 个月vs. 26.3个月)、PSS 1~3分(39.0个月 vs. 21.9个月)、低/高级别病理类型(41.5/40.9 个月vs. 20.1个月)、KPS≥80分(41.5 个月vs. 23.9个月)、无淋巴结转移(35.5 个月vs. 17.1个月)、Ki-67<50%(46.4 个月vs. 20.8个月)的患者中位生存时间延长(P<0.05)。5年生存预后分析中,单因素分析显示以下5个因素与5年生存率有关:PSS评分(P=0.021)、既往腹腔化疗(P=0.008)、病理类型(P=0.004)、淋巴结转移(P=0.008)和Ki-67表达程度(P=0.003)。多因素分析显示出以下3个影响5年生存的独立预后因素:既往腹腔化疗(HR=0.458,95%CI:0.253~0.827,P=0.010)、淋巴结转移(HR=2.879,95%CI:1.345~6.163,P=0.006)、Ki-67≥50%(HR=2.502,95%CI:1.418~4.417,P=0.002)。 结论 PMP非规范化治疗现象较普遍,误治时间长,淋巴结转移及Ki-67高表达是独立不良预后因素,CRS+HIPEC术前腹腔灌注化疗可能为PMP的治疗提供新的方向。 Abstract:Objective To analyze diagnosis and treatment status, natural history, and prognostic factors of patients with pseudomyxoma peritonei (PMP) based on real-world data. Methods Real-world data of patients with PMP with a complete natural history, including clinicopathological characteristics, non-standardized treatment (time of misdiagnosis, time of mistreatment, previous anti-tumor treatment), cytoreductive surgery + hyperthermic intraperitoneal chemotherapy (CRS+HIPEC) treatment [operation time, intraoperative blood transfusion, peritoneal cancer index (PCI) score, completeness of cytoreduction (CC) score, number of organs removed, number of peritoneal resection areas, serious adverse events (SAEs)], and follow-up until patient death, were retrospectively analyzed at Beijing Shijitan Hospital, Capital Medical University, from February 2009 to July 2020. The Kaplan-Meier method was used to draw survival curves, and the Log-rank test was used to compare between groups. Prognostic factors affecting survival were analyzed using univariate and multivariate analyses with a Cox proportional hazards regression model. Results : Overall, 94 patients were included, among whom 57 (60.6%) were male and 37(39.4%) were female; the median age of the patients was 54 (24–76) years. Fifty-nine (62.8%) patients previously received antitumor therapy. The median misdiagnosis time was 0.8 (0–62.5) months, and the median time of mistreatment was 15.3 (0–214.8) months. All patients received CRS+HIPEC treatment. The median operation time was 10.1 (4.8–16.5) hours, the mean number of organ resection was 2 (0–8), and the number of median peritonectomy was 5 (0–9). The PCI score was 32 (3–39) points, the CC score was 2–3 in 80.9% (76/94) of patients, and the SAE incidence rate was 35.1% (33/94). The median overall survival of 94 patients was 30.8 (2.4–218.4) months, and the 1-, 2-, 3-, and 5-year survival rates were 96.8%, 63.8%, 44.7%, and 23.4%, respectively. Stratified analysis showed that the median survival time of patients with previous intraperitoneal chemotherapy (46.5 months vs. 26.3 months), PSS 1–3 points (39.0 months vs. 21.9 months), low-/high-grade pathology (41.5/40.9 months vs. 20.1 months), KPS ≥80 (41.5 months vs. 23.9 months), no lymph node metastasis (35.5 months vs. 17.1 months), or Ki-67<50% (46.4 months vs. 20.8 months) was prolonged (P<0.05). Univariate analysis showed that the following five factors were related to 5-year survival rates: PSS score (P=0.021), previous intraperitoneal chemotherapy (P=0.008), pathological type (P=0.004), lymph node metastasis (P=0.008), and Ki-67 expression (P=0.003). Multivariate analysis revealed that the following three independent prognostic factors affected 5-year survival rates: previous intraperitoneal chemotherapy (HR=0.458, 95%CI: 0.253–0.827, P=0.010), lymph node metastasis (HR=2.879, 95%CI: 1.345–6.163, P=0.006), and Ki-67≥50% (HR=2.502, 95%CI: 1.418–4.417, P=0.002). Conclusions : Non-standardized treatment of PMP is common and long-standing in the real world. However, preoperative intraperitoneal infusion chemotherapy may provide a new treatment direction for PMP. -
表 1 患者的主要临床病理特征
临床病理特征 例数(%) 性别 男 57(60.6) 女 37(39.4) 首发症状 腹胀 80(85.1) 腹痛 10(10.6) 食欲减退 1(1.1) 腹泻 1(1.1) 排便困难 2(2.1) PSS评分(分) 0 29(30.8) 1 31(33.0) 2 31(33.0) 3 3(3.2) 既往静脉化疗 无 39(41.5) 有 55(58.5) 既往腹腔化疗 无 58(61.7) 有 36(38.3) 既往放疗 无 91(96.8) 有 3(3.2) 既往靶向治疗 无 77(81.9) 有 17(18.1) 病理分类 低级别 40(42.5) 高级别 31(33.0) 高级别伴印戒细胞 23(24.5) 术后辅助治疗 无 56(59.6) 有 38(40.4) 表 2 患者CRS+HIPEC治疗情况
临床指标 例数(%) CC(分) 0~1 18(19.1) 2~3 76(80.9) HIPEC药物 紫杉醇+铂类 63(67.0) 丝裂霉素+铂类 31(33.0) SAE 无 61(64.9) 有 33(35.1) 表 3 94例患者总生存时间分层分析
指标 中位生存时间(月) 95%CI Log-rank P 性别 1.611 0.204 男 29.2 18.209~40.191 女 39.6 25.507~59.633 年龄(岁) 0.072 0.788 ≥60 34.8 12.449~57.211 <60 30.8 22.110~34.590 BMI 1.918 0.166 ≥21 26.3 19.387~33.213 <21 35.9 24.612~47.328 KPS(分) 5.525 0.019 ≥80 41.5 34.130~48.870 <80 23.9 15.745~31.995 既往静脉化疗 0.130 0.718 无 29.8 17.809~41.791 有 34.3 25.311~43.229 既往靶向治疗 1.144 0.285 无 34.8 26.585~43.075 有 26.1 13.800~38.460 PCI(分) 0.041 0.840 <32 27.8 14.963~40.637 ≥32 30.8 23.100~38.500 CC(分) 0.783 0.376 0~1 26.1 21.764~30.496 2~3 34.8 27.109~42.551 SAE 1.322 0.250 无 30.8 22.382~39.218 有 34.3 19.144~49.396 HIPEC药物 0.309 0.578 紫杉醇+铂类 29.6 20.153~39.107 丝裂霉素+铂类 36.4 22.905~49.955 淋巴结转移 10.908 0.001 无 35.5 25.503~45.497 有 17.1 13.194~21.066 脉管癌栓 2.361 0.124 无 35.4 25.502~45.238 有 27.7 23.170~32.170 Ki-67(%) 5.504 0.019 <50 46.4 34.127~58.613 ≥50 20.8 17.794~23.866 术后辅助治疗 0.031 0.860 无 29.2 22.382~39.218 有 39.6 19.144~49.396 表 4 94例患者5年生存影响因素分析
预后因素 单因素分析 多因素分析 HR 95%CI P HR 95%CI P 既往腹腔化疗(无 vs. 有) 0.509 0.308~0.840 0.008 0.458 0.253~0.827 0.010 淋巴结转移(无 vs. 有) 3.247 1.554~6.784 0.002 2.879 1.345~6.163 0.006 Ki-67(<50% vs. ≥50%) 2.325 1.330~4.065 0.003 2.502 1.418~4.417 0.002 -
[1] Lin Y, Xu D, Li X, et al. Consensuses, and controversies on pseudomyxoma peritonei: a review of the published consensus statements and guidelines[J]. Orphanet J Rare Dis, 2021, 16(1):85-101. [2] Lin Y, Ma R, Li Y. The biological basis and function of GNAS mutation in pseudomyxoma peritonei: a review[J]. J Cancer Res Clin Oncol, 2020, 146(9):2179-2188. doi: 10.1007/s00432-020-03321-8 [3] Li Y, Zhou Y, Liang H, et al. Chinese expert consensus on cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal malignancies[J]. World J Gastroenterol, 2016, 22(30):6906-6916. [4] Li X, Ma R, Ji Z, et al. Perioperative safety after cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy for pseudomyxoma peritonei from appendiceal origin: experience on 254 patients from a single center[J]. Euro J Surg Oncol, 2020, 46(4):600-606. [5] Kozman MA, Fisher OM, Rebolledo BA J, et al. CA 19-9 to peritoneal carcinomatosis index (PCI) ratio is prognostic in patients with epithelial appendiceal mucinous neoplasms and peritoneal dissemination undergoing cytoreduction surgery and intraperitoneal chemotherapy[J]. Euro J Surg Oncol, 2017, 43(12):2299-2307. doi: 10.1016/j.ejso.2017.09.009 [6] 李雁,许洪斌,彭正,等.肿瘤细胞减灭术加腹腔热灌注化疗治疗腹膜假黏液瘤专家共识[J].中华医学杂志,2019,99(20):1527-1535. doi: 10.3760/cma.j.issn.0376-2491.2019.20.003 [7] Carr N. J. ; Cecil T. D. ; Mohamed F. Peritoneal Surface Oncology Group I. A consensus for classification and pathologic reporting of pseudomyxoma peritonei and associated appendiceal neoplasia: The results of the peritoneal surface oncology group international (psogi) modified delphi process[J]. Am J Surg Pathol, 2016, 40(1):14-26. [8] Li X, Yu Y, An S, et al. Impacts of prior surgical score on the eficacy and safety of cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy for pseudomyxoma peritonei[J]. Chin J Gen Surg, 2020, 35(10):782-787. [9] Mittal R, Chandramohan A, Moran B. Pseudomyxoma peritonei: natural history and treatment[J]. Int J Hyperther, 2017, 33(5):511-519. doi: 10.1080/02656736.2017.1310938 [10] Dayal S, Taflampas P, Riss S, et al. Complete cytoreduction for pseudomyxoma peritonei is optimal but maximal tumor debulking may be beneficial in patients in whom complete tumor removal cannot be achieved[J]. Dis Colon Rectum, 2013, 56(12):1366-1372. doi: 10.1097/DCR.0b013e3182a62b0d [11] Ma R, Xia A, Zhai X, et al. A single-center clinical analysis of 65 cases of pseudomyxoma peritonei from appendiceal origin in the early stage[J]. Chin J Oncol, 2019, 41(9):698-702. [12] Youssef H, Newman C, Chandrakumaran K, et al. Operative findings, early complications, and long-term survival in 456 patients with pseudomyxoma peritonei syndrome of appendiceal origin[J]. Dis Colon Rectum, 2011, 54(3):293-299. doi: 10.1007/DCR.0b013e318202f026 [13] Chua TC, Moran BJ, Sugarbaker PH, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic Intraperitoneal chemotherapy[J]. J Clin Oncol, 2012, 30(20):2449-2456. doi: 10.1200/JCO.2011.39.7166 [14] Merrell DS, McAvoy TJ, King MC, et al. Pre- and post-operative antibiotics in conjunction with cytoreductive surgery and heated intraperitoneal chemotherapy (HIPEC) should be considered for pseudomyxoma peritonei (PMP) treatment[J]. Euro J Surg Oncol, 2019, 45(9):1723-1726. doi: 10.1016/j.ejso.2019.01.223 [15] Ma R, Wang B, Zhai X, et al. Management and prognostic prediction of appendiceal mucinous adenocarcinoma with peritoneal metastasis: a single center study in China[J]. BMC Cancer, 2020, 20(1):1-6. doi: 10.1186/s12885-019-6169-0 [16] Yan F, Lin Y, Zhao H, et al. Pathological prognostic factors of pseudomyxoma peritonei[J]. Chin J Pathol, 2019, 48(7):543-549. [17] Fujiwara Y, Takiguchi S, Nakajima K, et al. Neoadjuvant Intraperitoneal and systemic chemotherapy for gastric cancer patients with peritoneal dissemination[J]. Ann Surg Oncol, 2011, 18(13):3726-3731. doi: 10.1245/s10434-011-1770-8 [18] Yonemura Y, Prabhu A, Sako S, et al. Long term survival after cytoreductive surgery combined with perioperative chemotherapy in gastric cancer patients with peritoneal metastasis[J]. Cancers, 2020, 12(1):116-126. doi: 10.3390/cancers12010116 [19] Prabhu A, Brandl A, Wakama S, et al. Neoadjuvant intraperitoneal chemotherapy in patients with pseudomyxoma peritonei-a novel treatment approach[J]. Cancers, 2020, 12(8):2212-2223. doi: 10.3390/cancers12082212 [20] Padmanabhan N, Ishibashi H, Nishihara K, et al. Complete pathological response of high grade appendicular neoplasm induced pseudomyxoma peritonei (PMP) after neoadjuvant intra-peritoneal chemotherapy: a case report[J]. Int J Surg Case Rep, 2020, 72:117-121. doi: 10.1016/j.ijscr.2020.05.072 [21] Sugarbaker Paul H, Van der Speeten Kurt, Stuart O Anthony. Pharmacologic rationale for treatments of peritoneal surface malignancy from colorectal cancer[J]. World J Gastrointest Oncol, 2010, 2(1):19-30. [22] Ceelen Wim, Braet Helena, van Ramshorst Gabrielle, et al. Intraperitoneal chemotherapy for peritoneal metastases: an expert opinion[J]. Expert Opin Drug Deliv, 2020, 17(4):511-522. doi: 10.1080/17425247.2020.1736551 [23] Esquivel J, Vidal-Jove J, Steves MA, et al. Morbidity and mortality of cytoreductive surgery and intraperitoneal chemotherapy[J]. Surgery, 1993, 113:631-636. [24] Blackham AU, Swett K, Eng C, et al. Perioperative systemic chemotherapy for appendiceal mucinous carcinoma peritonei treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: perioperative chemo for appendiceal MCP[J]. J Surg Oncol, 2014, 109(7):740-745. doi: 10.1002/jso.23547 [25] Huang Y, Alzahrani NA, Liauw W, et al. Early postoperative intraperitoneal chemotherapy for low-grade appendiceal mucinous neoplasms with pseudomyxoma peritonei: Is it beneficial[J]. Ann Surg Oncol, 2017, 24(1):176-183. doi: 10.1245/s10434-016-5529-0 -



 下载:
下载:


