Efficacy of low-dose apatinib in treating reactive cutaneous capillary endothelial proliferation induced by camrelizumab
-
摘要:
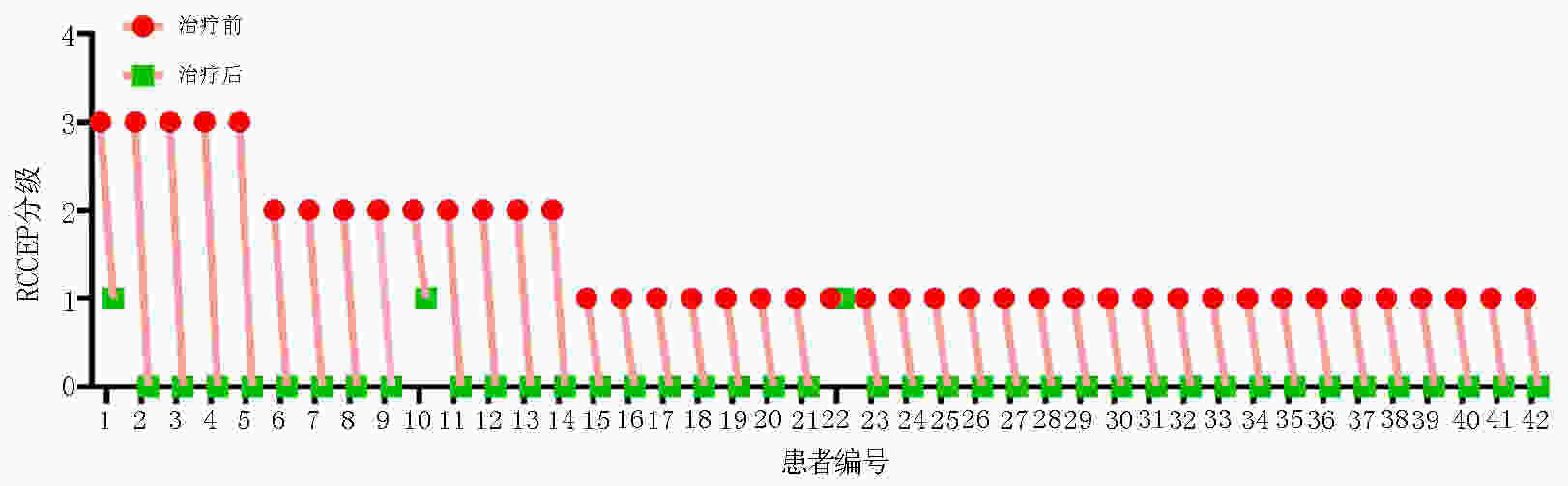
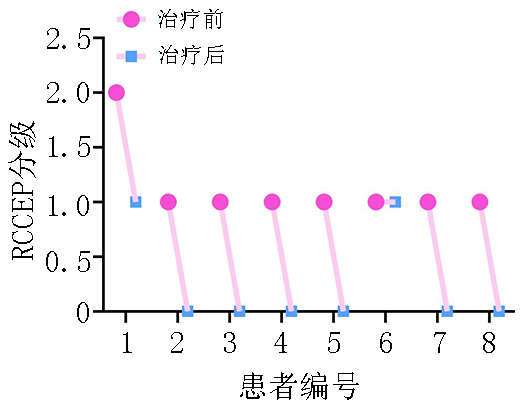
目的 评价低剂量阿帕替尼治疗卡瑞利珠单抗致反应性毛细血管增生症(reactive cutaneous capillary endothelial proliferation,RCCEP)的疗效和安全性。 方法 回顾性分析不同抗血管生成药物联合卡瑞利珠单抗治疗晚期实体肿瘤140例的RCCEP发生率。观察250 mg和125 mg阿帕替尼两个剂量组治疗50例单药卡瑞利珠单抗诱发RCCEP的疗效和安全性。 结果 140例晚期实体瘤使用卡瑞利珠单抗联合不同抗血管生成药物的RCCEP发生率:贝伐珠单抗组为93.3%,安罗替尼组为72.7%,呋喹替尼组为47.6%,瑞戈非尼组为70.6%,阿帕替尼组为14.0%。阿帕替尼较其它抗血管生成药物显著降低RCCEP发生率:贝伐珠单抗组 vs. 阿帕替尼组(P<0.000 1);安罗替尼组 vs. 阿帕替尼组(P<0.000 1);呋喹替尼组 vs. 阿帕替尼组(P=0.005);瑞戈非尼组 vs. 阿帕替尼组(P<0.000 1)。观察50例晚期实体瘤不同剂量阿帕替尼治疗卡瑞利珠单抗诱发RCCEP的疗效和安全性,结果提示42例250 mg阿帕替尼治疗组有效率为97.6%,8例125 mg阿帕替尼治疗组有效率为87.5%;两个剂量组均未出现≥G3的治疗相关不良事件。 结论 阿帕替尼较其它抗血管生成药物更显著降低卡瑞利珠单抗的RCCEP发生率,低剂量阿帕替尼可有效安全地缓解RCCEP。 -
关键词:
- 低剂量阿帕替尼 /
- 卡瑞利珠单抗 /
- 反应性毛细血管增生症 /
- 疗效 /
- 安全性
Abstract: Objective: To evaluate the efficacy and safety of low-dose apatinib in treating reactive cutaneous capillary endothelial proliferation (RCCEP) induced by camrelizumab. Methods: RCCEP incidence was retrospectively analyzed in 140 patients with advanced solid tumors treated with different types of antiangiogenic drugs combined with camrelizumab. The efficacy and safety of 125 and 250 mg apatinib were analyzed for treating camrelizumab-induced RCCEP in 50 patients with advanced solid tumors. Results: RCCEP incidence in 140 patients with advanced solid tumors treated with camrelizumab combined with different types of antiangiogenic drugs was retrospectively analyzed. The results showed that the incidence of camrelizumab-induced RCCEP in the bevacizumab, anlotinib, fruquintinib, regofinib, and apatinib groups was 93.3%, 72.7%, 47.6%, 70.6%, and 14.0%, respectively. Apatinib significantly reduced RCCEP incidence compared with other antiangiogenic drugs: bevacizumab group vs. apatinib group (P<0.000 1); anlotinib group vs. apatinib group (P<0.0001); fruquintinib group vs. apatinib group (P=0.005); and regofinib group vs. apatinib group (P<0.000 1). The efficacy and safety of different low-dose apatinib were observed in 50 patients with RCCEP and advanced solid tumors. The total effective rate of 250 mg apatinib was 97.6% when treating 42 patients with RCCEP, while that of 125 mg apatinib was 87.5% when treating eight patients with RCCEP. There were no ≥grade 3 treatment-related adverse events in both patient groups treated with 125 and 250 mg apatinib. Conclusions: Apatinib could significantly reduce the incidence of camrelizumab-induced RCCEP compared with other antiangiogenic drugs. Low-dose apatinib could effectively and safely alleviate RCCEP. -
表 1 卡瑞利珠单抗联合不同类型抗血管生成药物治疗的患者临床特征 n(%)
临床特征 总计(n=140) 卡瑞利珠单抗+
贝伐珠单抗(n=30)卡瑞利珠单抗+
安罗替尼(n=22)卡瑞利珠单抗+
呋喹替尼(n=21)卡瑞利珠单抗+
瑞戈非尼(n=17)卡瑞利珠单抗+
阿帕替尼(n=50)性别 男 44(31.4) 5(3.6) 11(7.9) 3(2.1) 7(5.0) 18(12.9) 女 96(68.6) 25(17.9) 11(7.9) 18(12.9) 10(7.1) 32(22.9) ECOG评分(分) 0~1 88(62.9) 17(12.1) 9(6.4) 15(10.7) 12(8.6) 35(25.0) 2 52(37.1) 13(9.3) 13(9.3) 6(4.3) 5(3.6) 15(10.7) 实体肿瘤类型 非小细胞肺癌 46(32.9) 30(21.4) 16(11.4) 0(0.0) 0(0.0) 0(0.0) 结直肠癌 48(34.3) 0(0) 0(0) 21(15) 17(12.1) 10(7.1) 胃癌 20(14.3) 0(0) 0(0) 0(0) 0(0) 20(14.3) 食管癌 11(7.9) 0(0) 6(4.3) 0(0) 0(0) 5(3.6) 肝癌 15(10.7) 0(0) 0(0) 0(0) 0(0) 15(10.7) 转移灶累及器官数目(个) 1~2 60(42.9) 10(7.1) 12(8.6) 10(7.1) 6(4.3) 22(15.7) ≥3 80(57.1) 20(14.2) 10(7.1) 11(7.9) 11(7.9) 28(20.0) 既往系统治疗线数 1 2(1.4) 2(1.4) 0(0) 0(0) 0(0) 0(0) 2 49(35.0) 28(20.0) 6(4.3) 0(0) 0(0) 15(10.7) ≥3 10(77.9) 0(0) 16(72.7) 21(15.0) 17(12.1) 35(25.0) 表 2 卡瑞利珠单抗联合不同类型抗血管生成药物的RCCEP发生率 n(%)
治疗方案 总计 RCCEP G1~2级 G3~4级 所有分级 卡瑞利珠单抗+贝伐珠单抗 30 25(83.3) 3(10.0) 28(93.3) 卡瑞利珠单抗+安罗替尼 22 15(68.2) 1(4.5) 16(72.7) 卡瑞利珠单抗+呋喹替尼 21 9(43.8) 1(4.8) 10(47.6) 卡瑞利珠单抗+瑞戈非尼 17 11(64.7) 1(5.9) 12(70.6) 卡瑞利珠单抗+阿帕替尼 50 7(14.0) 0(0) 7(14.0) 表 3 不同剂量阿帕替尼治疗RCCEP的患者临床特征 n(%)
临床特征 总计(n=50) 阿帕替尼 250 mg组(n=42) 125 mg组(n=8) 性别 男 33(66.0) 28(56.0) 5(10.0) 女 17(34.0) 14(28.0) 3(6.0) ECOG评分(分) 0~1 25(50.0) 22(44.0) 3(6.0) 2 25(50.0) 20(40.0) 5(10.0) 实体肿瘤类型 非鳞非小细胞肺癌 17(34.0) 14(28.0) 3(6.0) 肝细胞癌 10(20.0) 8(16.0) 2(4.0) 食管鳞癌 12(24.0) 10(20.0) 2(4.0) 胃癌 5(10.0) 5(10.0) 0(0) 结直肠癌 6(12.0) 5(10.0) 1(2.0) 表 4 不同低剂量阿帕替尼治疗相关的不良反应 n(%)
不良反应 阿帕替尼250 mg组(n=42) 阿帕替尼125 mg组(n=8) G1 G2 ≥G3 G1 G2 ≥G3 高血压 5(11.9) 2(4.8) 0(0) 1(12.5) 1(12.5) 0(0) 疲乏 2(4.8) 0(0) 0(0) 1(12.5) 0(0) 0(0) 蛋白尿 3(7.1) 1(2.4) 0(0) 0(0) 0(0) 0(0) 手足综合征 4(9.5) 2(4.8) 0(0) 0(0) 0(0) 0(0) 声音嘶哑 2(4.8) 0(0) 0(0) 0(0) 0(0) 0(0) 口腔黏膜炎 1(2.4) 0(0) 0(0) 0(0) 0(0) 0(0) 食欲减退 3(7.1) 0(0) 0(0) 1(12.5) 0(0) 0(0) 腹泻 2(4.8) 0(0) 0(0) 0(0) 0(0) 0(0) 血小板减少 2(4.8) 0(0) 0(0) 0(0) 0(0) 0(0) 白细胞减少 3(7.1) 0(0) 0(0) 0(0) 0(0) 0(0) 血红蛋白减少 3(7.1) 0(0) 0(0) 0(0) 0(0) 0(0) -
[1] Chen JD, Quan M, Chen ZQ, et al. Camrelizumab in advanced or metastatic solid tumour patients with DNA mismatch repair deficient or microsatellite instability high: an open-label prospective pivotal trial[J]. J Cancer Res Clin Oncol, 2020, 146(10):2651-2657. doi: 10.1007/s00432-020-03251-5 [2] Markham A, Keam SJ. Camrelizumab: first global approval[J]. Drugs, 2019, 79(12):1355-1361. doi: 10.1007/s40265-019-01167-0 [3] Hwang SJ, Carlos G, Wakade D, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: A single-institution cohort[J]. J Am Acad Dermatol, 2016, 74(3):455-461. doi: 10.1016/j.jaad.2015.10.029 [4] Wang F, Qin SK, Sun XC, et al. Reactive cutaneous capillary endothelial proliferation in advanced hepatocellular carcinoma patients treated with camrelizumab: data derived from a multicenter phase 2 trial[J]. J Hematol Oncol, 2020, 13(1):47. doi: 10.1186/s13045-020-00886-2 [5] Teng Y, Guo RF, Sun JF, et al. Reactive capillary hemangiomas induced by camrelizumab (SHR-1210), an anti-PD-1 agent[J]. Acta Oncol, 2019, 58(3):388-389. doi: 10.1080/0284186X.2019.1567935 [6] Zhou CC, Wang YN, Zhao J, et al. Efficacy and biomarker analysis of camrelizumab in combination with apatinib in patients with advanced nonsquamous NSCLC previously treated with chemotherapy[J]. Clin Cancer Res, 2021, 27(5):1296-1304. doi: 10.1158/1078-0432.CCR-20-3136 [7] Ortins-Pina A, Soares-de-Almeida L, Caroli U, et al. Prurigiform angiomatosis: reactive angioproliferation in the skin and vascular endothelial growth factors[J]. Am J Dermatopathol, 2020, 42(1):29-34. doi: 10.1097/DAD.0000000000001452 [8] Scott LJ. Apatinib: A review in advanced gastric cancer and other advanced cancers[J]. Drugs, 2018, 78(7):747-758. doi: 10.1007/s40265-018-0903-9 [9] 秦叔逵,马军,李进,等.卡瑞利珠单抗致反应性皮肤毛细血管增生症临床诊治专家共识[J].临床肿瘤学杂志,2020,25(9):840-848. doi: 10.3969/j.issn.1009-0460.2020.09.012 [10] Lickliter JD, Gan HK, Voskoboynik M, et al. A first-in-human dose finding study of camrelizumab in patients with advanced or metastatic cancer in Australia[J]. Drug Des Devel Ther, 2020, 14:1177-1189. doi: 10.2147/DDDT.S243787 [11] Song YQ, Wu JQ, Chen XC, et al. A single-arm, multicenter, phase Ⅱ study of camrelizumab in relapsed or refractory classical Hodgkin lymphoma[J]. Clin Cancer Res, 2019, 25(24):7363-7369. doi: 10.1158/1078-0432.CCR-19-1680 [12] Xu JM, Zhang Y, Jia R, et al. Anti-PD-1 antibody SHR-1210 combined with apatinib for advanced hepatocellular carcinoma, gastric, or esophagogastric junction cancer: an open-label, dose escalation and expansion study[J]. Clin Cancer Res, 2019, 25(2):515-523. doi: 10.1158/1078-0432.CCR-18-2484 [13] Peng Z, Wei J, Wang F, et al. Camrelizumab combined with chemotherapy followed by camrelizumab plus apatinib as first-line therapy for advanced gastric or gastroesophageal junction adenocarcinoma[J]. Clin Cancer Res, 2021, 27(11):3069-3078. doi: 10.1158/1078-0432.CCR-20-4691 [14] Jiang FE, Zhang HJ, Yu CY, et al. Efficacy and safety of regorafenib or fruquintinib plus camrelizumab in patients with microsatellite stable and/or proficient mismatch repair metastatic colorectal cancer: an observational pilot study[J]. Neoplasma, 2021, 68(4):861-866. [15] Li WH, Wei ZG, Yang X, et al. Salvage therapy of reactive capillary hemangiomas: Apatinib alleviates the unique adverse events induced by camrelizumab in non-small cell lung cancer[J]. J Cancer Res Ther, 2019, 15(7):1624-1628. doi: 10.4103/jcrt.JCRT_997_19 [16] Liu JQ, Liu Q, Li Y, et al. Efficacy and safety of camrelizumab combined with apatinib in advanced triple-negative breast cancer: an open-label phase II trial[J]. J Immunother Cancer, 2020, 8(1):e000696. [17] Wang DX, Yang X, Long JY, et al. The efficacy and safety of apatinib plus camrelizumab in patients with previously treated advanced biliary tract cancer: a prospective clinical study[J]. Front Oncol, 2021, 11:646979. -




 下载:
下载: