Clinicopathological characteristics and prognostic analysis of gastric cancer patientswith positive resection margin after radical surgery
-
摘要:
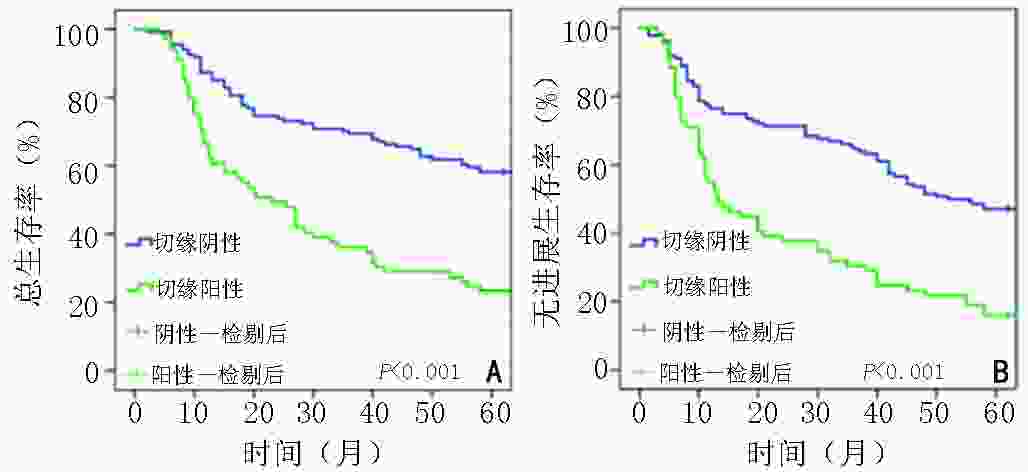
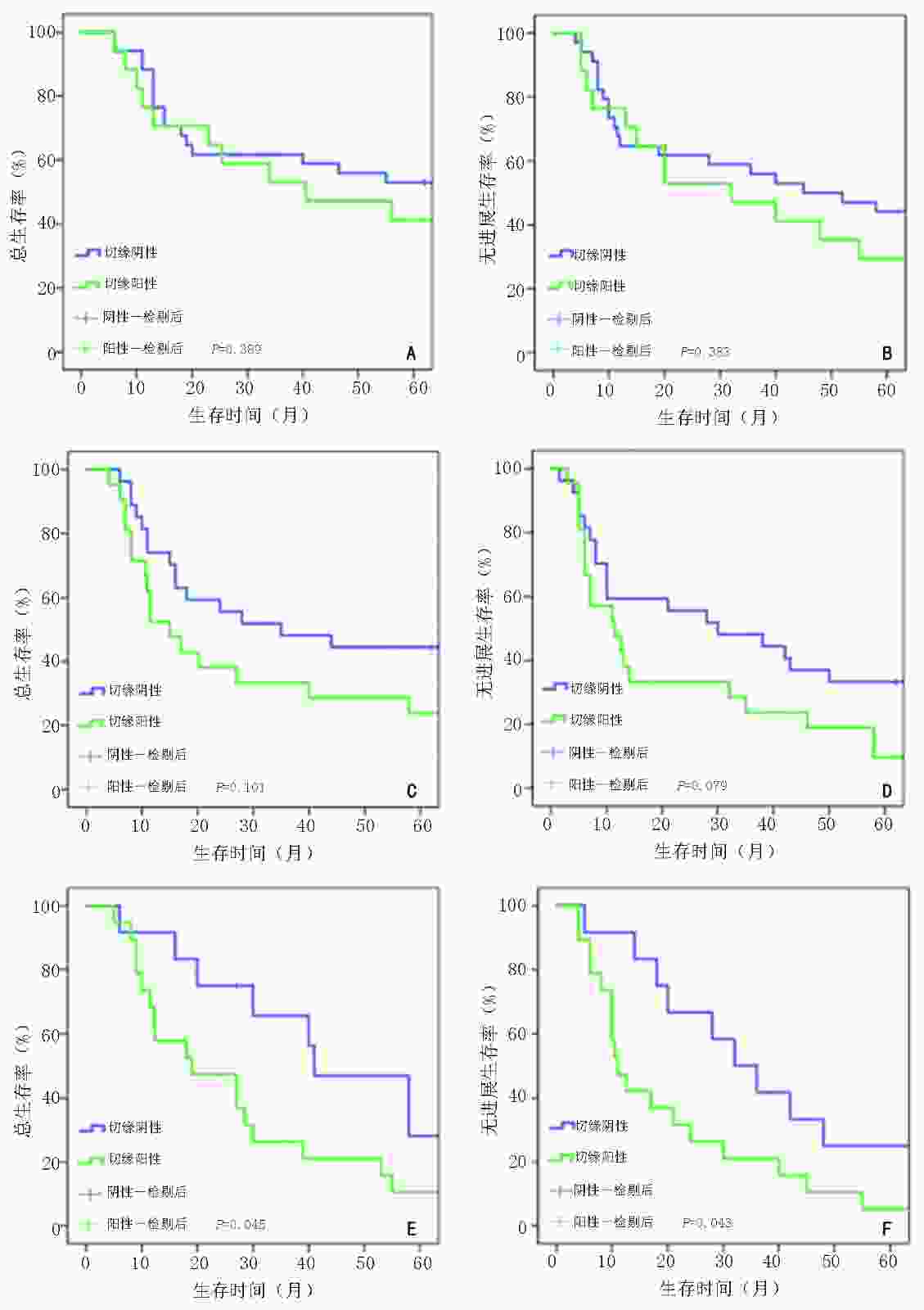
目的 探讨胃癌根治术后切缘阳性患者的临床病理特征及其对预后影响。 方法 回顾性分析河北医科大学第四医院2011年1月至2016年1月收治的胃癌根治术后切缘阳性患者的临床病理资料。按1∶2随机数法选取同期收治的切缘阴性患者,比较阳性和阴性切缘患者的一般临床病理学特征及预后情况。 结果 共纳入73例切缘阳性患者,与同期纳入的146例切缘阴性病例比较,阳性组的肿瘤直径更大、更多位于贲门或全胃,组织学类型更差、Lauren分型趋于弥漫型、Borrmann分型多为Ⅲ~Ⅳ型、肿瘤浸润深度以T4a~4b为主、pTNM分期更晚,脉管浸润率及淋巴结转移率也更高,同时术者经验、手术方式的差异也与阳性切缘发生有关(均P<0.05)。全组共有205例患者获得完整随访,两组患者5年总生存(overall survival,OS)率及无进展生存(progression-free survival,PFS)率均有显著性差异(23.19% vs. 58.82%,15.94% vs. 47.06%,均P<0.001)。Cox多因素分析显示,切缘状态(P=0.012)、pTNM分期(P=0.023)及术后综合治疗(化疗/化疗联合放疗)(P<0.001)是影响胃癌预后的独立因素。 结论 胃癌根治术后切缘状态与多种临床病理特征相关,切缘阳性患者预后较差。 Abstract:Objective To investigate the clinicopathological characteristics of patients with positive resection margin after radical surgery for gastric cancer and the impact of positive margin on prognosis. Methods We retrospectively collected the clinicopathological data of gastric cancer patients with positive resection margins admitted to Fourth Hospital of Hebei Medical University for radical gastrectomy from January 2011 to January 2016. Patients with negative resection margins admitted at the same period were selected using the random number method (ratio, 2∶1), and the general clinicopathological characteristics and prognosis of patients with positive and negative margins were compared. Results In total, 73 patients with positive resection margins and 146 with negative resection margins were included. Compared to patients with negative margins, those with positive margins had larger tumor sizes, greater number of tumors in the cardia or whole stomach, worse histological types, advanced tumor pTNM stages, and higher rates of vascular invasion and lymph node metastasis; their Lauren type tended to be diffuse, Borrmann type tended to be types Ⅲ–Ⅳ, and tumor invasion depth mostly T4a-4b. The difference in operative experience and surgical methods was also associated with positive margin (all P<0.05). A total of 205 patients were followed up completely. There were significant differences in 5-year overall survival (OS) and progression-free survival (PFS) between the two groups (23.19% vs. 58.82%, 15.94% vs. 47.06%, all P<0.001). Cox multivariate analysis showed that the resection margin status (P=0.012), tumor pTNM stage (P=0.023), and postoperative comprehensive treatment (chemotherapy/chemoradiotherapy, P<0.001) were independent factors for the prognosis of gastriccancer. Conclusions The resection margin status after radical gastric cancer is related to various clinicopathological characteristics, and patients with positive margins have a poor prognosis. -
Key words:
- gastric cancer /
- radical surgery /
- positive resection margin /
- risk factors /
- prognostic analysis
-
表 1 胃癌患者临床病理特征与切缘状态关系的单因素分析
临床病理特征 切缘阳性(n=73) 切缘阴性(n=146) χ2 P 性别 2.190 0.139 男 52(71.23) 117(80.14) 女 21(28.77) 29(19.86) 年龄(岁) 0.932 0.334 <60 28(38.36) 66(45.21) ≥60 45(61.64) 80(54.79) 肿瘤直径(cm) 16.946 <0.001 <5 20(27.40) 83(56.85) ≥5 53(72.60) 63(43.15) 肿瘤位置 21.440 <0.001 贲门 35(47.94) 52(35.62) 胃体 7(9.59) 19(13.01) 胃窦 11(15.07) 60(41.10) 全胃 20(27.40) 15(10.27) 切缘距离(cm) 12.578 <0.001 <3 46(63.01) 55(37.67) ≥3 27(36.99) 91(62.33) 组织学类型 15.144 <0.001 低-未分化 60(82.19) 81(55.48) 高-中分化 13(17.81) 65(44.52) Borrmann分型(型) 27.175 <0.001 Ⅰ 3(4.12) 12(8.22) Ⅱ 9(12.33) 48(32.88) Ⅲ 39(53.41) 76(52.05) Ⅳ 22(30.14) 10(6.85) pT分期(期) 21.542 <0.001 T1~3 9(12.33) 39(26.71) T4a 45(61.64) 99(67.81) T4b 19(26.03) 8(5.48) pN分期(期) 17.522 0.001 N0 12(16.44) 58(39.73) N1 5(6.85) 12(8.22) N2 15(20.55) 33(22.60) N3 41(56.16) 43(29.45) pTNM分期(期) 18.010 <0.001 Ⅰ 4(5.48) 31(21.24) Ⅱ 8(10.96) 35(23.97) Ⅲ 61(83.56) 80(54.79) Lauren分型 33.241 <0.001 弥漫型 41(56.16) 29(19.86) 肠型 16(21.92) 83(56.85) 混合性 16(21.92) 34(23.29) 脉管瘤栓 11.634 0.001 有 31(43.84) 30(20.55) 无 42(56.16) 116(79.45) 神经受侵 2.668 0.102 有 24(32.88) 33(22.60) 无 49(67.12) 113(77.40) 血清CEA(mg/mL) 0.107 0.744 ≤5 53(72.60) 109(74.66) >5 20(27.40) 37(25.34) 血清CA19-9(U/mL) 6.167 0.013 ≤30 51(69.86) 123(84.25) >30 22(30.14) 23(15.75) 血清CA72-4(U/mL) 6.272 0.012 ≤6.9 49(67.12) 120(82.19) >6.9 24(32.88) 26(17.81) 术者经验(例) 24.502 <0.001 ≥100 32(43.84) 113(77.40) <100 41(56.16) 33(22.60) 手术方式 4.469 0.035 开腹/腹腔镜辅助 33(45.21) 88(60.27) 完全腹腔镜 40(54.79) 58(39.73) 术前新辅助化疗 5.230 0.022 是 12(16.44) 45(30.82) 否 61(83.56) 101(69.18) ()内单位为% 表 2 胃癌患者临床病理特征与切缘状态关系的Logistic回归多因素分析
临床因素 β SE OR 95%CI P 肿瘤直径(cm)(≥5 vs. <5 ) 0.691 0.442 1.995 0.838~4.746 0.118 肿瘤位置(贲门或全胃 vs. 胃窦或胃体) 1.181 0.427 3.257 1.410~7.524 0.006 切缘距离(cm)(<3 vs. ≥3 ) 1.016 0.444 2.763 1.157~6.596 0.022 组织学类型(低、未分化 vs. 高、中分化) 1.188 0.491 3.282 1.254~8.589 0.015 Borrmann分型(型)(Ⅲ~Ⅳ vs. Ⅰ~Ⅱ) 0.468 0.505 1.596 0.594~4.292 0.354 pT分期(期)(T4 vs. T1~T3) 0.468 0.770 1.598 0.353~7.223 0.543 pN分期期(期)(N+ vs. N0) 0.186 0.935 1.205 0.193~7.508 0.842 pTNM分期(期)(Ⅲ vs. Ⅰ~Ⅱ) 0.147 1.012 1.159 0.159~8.417 0.884 Lauren分型(弥漫型 vs. 混合型和肠型) 1.485 0.436 4.415 1.877~10.386 0.001 脉管瘤栓(有 vs. 无) 0.167 0.441 1.181 0.498~2.802 0.705 血清CA19-9(U/mL)(>30 vs. ≤30) 0.412 0.514 1.510 0.552~4.135 0.422 血清CA72-4(U/mL)(>6.9 vs. ≤6.9) 0.917 0.473 2.503 0.991~6.321 0.052 术者经验(例)(<100 vs. ≥100) 2.012 0.469 7.481 2.984~18.752 <0.001 手术方式(腹腔镜 vs. 开腹) 1.008 0.419 2.739 1.205~6.226 0.016 术前新辅助化疗(是 vs. 否) −1.149 0.514 0.317 0.116~0.868 0.025 表 3 影响胃癌根治术后患者预后的Cox回归多因素分析
临床病理因素 β SE HR 95%CI P 组织学类型(低-未分化 vs. 高-中分化) 0.047 0.244 1.049 0.650~1.692 0.846 Borrmann分型(型)(Ⅲ~Ⅳ vs. Ⅰ~Ⅱ) 0.059 0.277 1.061 0.616~1.828 0.831 pT分期(期)(T4a~T4b vs. T1~3) 0.287 0.548 1.332 0.455~3.898 0.601 pN分期(期)(N+ vs. N0) 0.225 0.501 1.252 0.469~3.346 0.654 pTNM分期(期)(Ⅲ vs. Ⅰ~Ⅱ) 0.939 0.414 2.557 1.136~5.759 0.023 Lauren分型(弥漫型 vs. 肠型及混合型) 0.035 0.233 1.035 0.656~1.633 0.881 脉管瘤栓(有 vs. 无) 0.217 0.254 1.243 0.755~2.046 0.393 神经受侵(有 vs. 无) 0.118 0.244 1.125 0.697~1.816 0.628 肿瘤长径(cm)(≥5 vs. <5) 0.031 0.238 1.032 0.648~1.644 0.895 血清CA19-9(U/mL/)(>30 vs. ≤30) 0.034 0.249 1.034 0.634~1.686 0.893 血清CA72-4(U/mL/)(>6.9 vs. ≤6.9) 0.269 0.229 1.308 0.835~2.049 0.241 切缘状态(阳性 vs. 阴性) 0.644 0.256 1.905 1.153~3.142 0.012 术后综合化疗(放化疗 vs. 单纯化疗 vs. 未治疗) −1.442 0.367 0.236 0.115~0.485 <0.001 -
[1] 丁平安,杨沛刚,张志栋,等.系统性免疫炎症指数与胃癌根治术后患者预后的相关性研究[J].中华消化杂志,2021,41(8):534-540. doi: 10.3760/cma.j.cn311367-20201206-00690 [2] Lin YS, Zheng Y, Wang HL, et al. Global patterns and trends in gastric cancer incidence rates (1988-2012) and predictions to 2030[J]. Gastroenterology, 2021, 161(1):116-127. doi: 10.1053/j.gastro.2021.03.023 [3] Kakeji Y, Tsujitani S, Baba H, et al. Clinicopathologic features and prognostic significance of duodenal invasion in patients with distal gastric carcinoma[J]. Cancer, 1991, 68(2):380-384. doi: 10.1002/1097-0142(19910715)68:2<380::AID-CNCR2820680228>3.0.CO;2-A [4] Sun Z, Li DM, Wang ZN, et al. Prognostic significance of microscopic positive margins for gastric cancer patients with potentially curative resection[J]. Ann Surg Oncol, 2009, 16(11):3028-3037. doi: 10.1245/s10434-009-0624-0 [5] 陕飞, 李子禹, 张连海, 等. 国际抗癌联盟及美国肿瘤联合会胃癌TNM分期系统(第8版)简介及解读[J].中国实用外科杂志,2017,37(1):15-17. [6] Das M. Neoadjuvant chemotherapy: survival benefit in gastric cancer[J]. Lancet Oncol, 2017, 18(6):e307. doi: 10.1016/S1470-2045(17)30321-2 [7] Yu J, Huang CM, Sun YH, et al. Effect of laparoscopic vs open distal gastrectomy on 3-year disease-free survival in patients with locally advanced gastric cancer: The CLASS-01 randomized clinical trial[J]. JAMA, 2019, 321(20):1983-1992. doi: 10.1001/jama.2019.5359 [8] Kim W, Kim HH, Han SU, et al. Decreased morbidity of laparoscopic distal gastrectomy compared with open distal gastrectomy for stage Ⅰ gastric cancer: short-term outcomes from a multicenter randomized controlled trial (KLASS-01)[J]. Ann Surg, 2016, 263(1):28-35. [9] Katai H, Mizusawa J, Katayama H, et al. Short-term surgical outcomes from a phase Ⅲ study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage ⅠA/ⅠB gastric cancer: Japan Clinical Oncology Group Study JCOG0912[J]. Gastric Cancer, 2017, 20(4):699-708. doi: 10.1007/s10120-016-0646-9 [10] Andersson Y, Bergkvist L, Frisell J, et al. Long-term breast cancer survival in relation to the metastatic tumor burden in axillary lymph nodes[J]. Breast Cancer Res Treat, 2018, 171(2):359-369. doi: 10.1007/s10549-018-4820-0 [11] de Paul TR, Augestad KM, Kiran RP, et al. Management of the positive pathologic circumferential resection margin in rectal cancer: A national cancer database (NCDB) study[J]. Eur J Surg Oncol, 2021, 47(2):296-303. doi: 10.1016/j.ejso.2020.07.033 [12] Woo JW, Ryu KW, Park JY, et al. Prognostic impact of microscopic tumor involved resection margin in advanced gastric cancer patients after gastric resection.[J]. World J Surg, 2014, 38(7):439-446. [13] Lee JH, Ahn SH, Park DJ, et al. Clinical impact of tumor infiltration at the transected surgical margin during gastric cancer surgery[J]. J Surg Oncol, 2012, 106(6):772-776. doi: 10.1002/jso.23123 [14] Pedrazzani C, Marrelli D, Pacelli F, et al. Gastric linitis plastica: which role for surgical resection[J]? Gastric Cancer, 2012, 15(1):56-60. [15] Liang YX, Ding XW, Wang XN, et al. Prognostic value of surgical margin status in gastric cancer patients[J]. ANZ J Surg, 2015, 85(9):678-684. [16] Shin D, Park SS. Clinical importance and surgical decision-making regarding proximal resection margin for gastric cancer[J]. World J Gastrointest Oncol, 2013, 5(1):4-11. doi: 10.4251/wjgo.v5.i1.4 [17] Spicer J, Benay C, Lee L, et al. Diagnostic accuracy and utility of intraoperative microscopic margin analysis of gastric and esophageal adenocarcinoma[J]. Ann Surg Oncol, 2014, 21(8):2580-2586. doi: 10.1245/s10434-014-3669-7 [18] Matsusaka S, Nagareda T, Yamasaki H, et al. Immunohistochemical evaluation for intraoperative rapid pathological assessment of the gastric margin[J]. World J Surg, 2003, 27(6):715-718. doi: 10.1007/s00268-003-6792-3 [19] Tu RH, Lin JX, Wang W, et al. Pathological features and survival analysis of gastric cancer patients with positive surgical margins: A large multicenter cohort study[J]. Eur J Surg Oncol, 2019, 45(12):2457-2464. [20] Rhome RM, Moshier E, Sarpel U, et al. Predictors of positive margins after definitive resection for gastric adenocarcinoma and impact of adjuvant therapies[J]. Int J Radiat Oncol Biol Phys, 2017, 98(5):1106-1115. doi: 10.1016/j.ijrobp.2017.03.041 -




 下载:
下载: