Expert consensus on the diagnosis and treatment of bone metastasis in patients with breast cancer
-
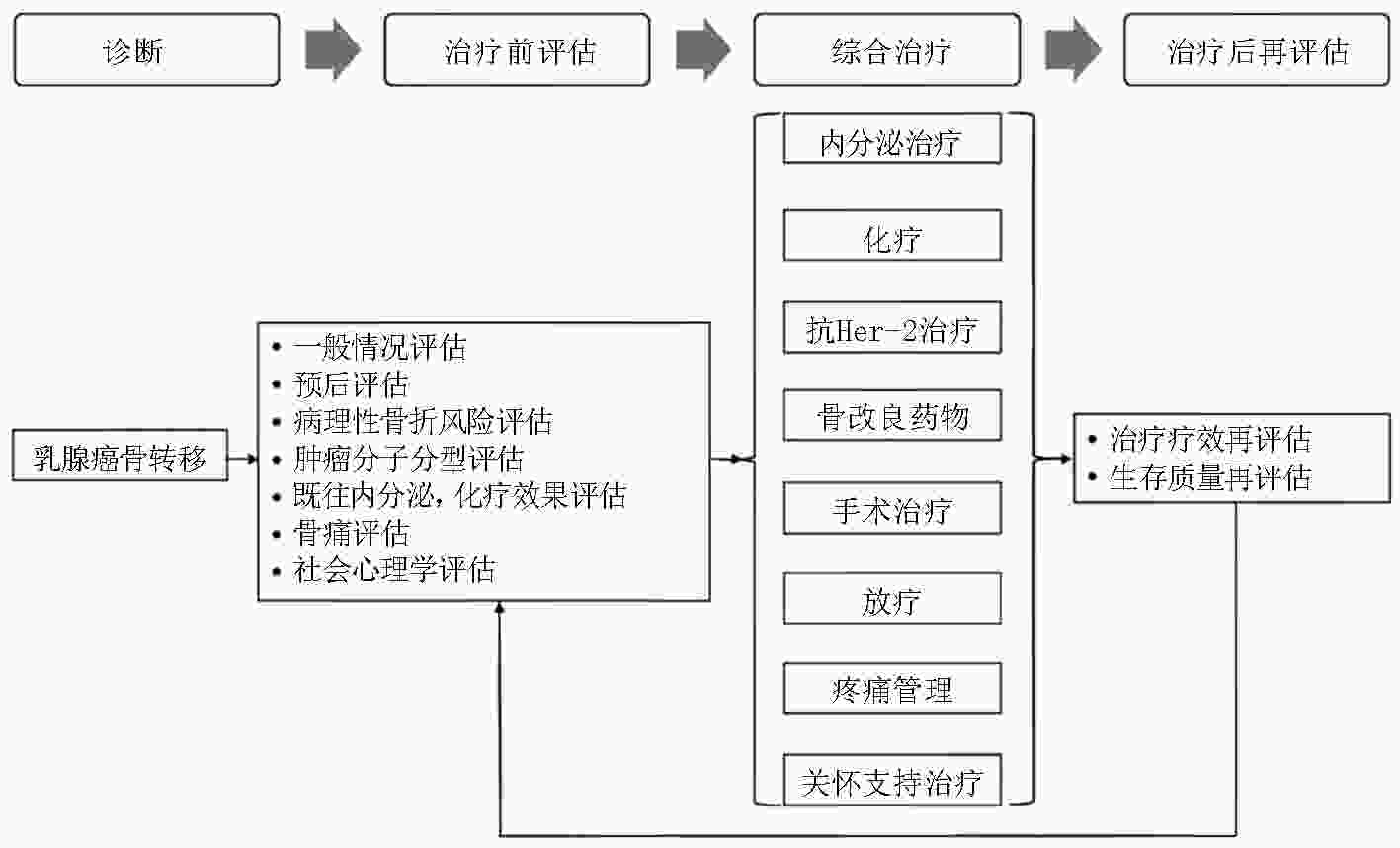
摘要: 骨骼是乳腺癌最常见的远处转移部位,约占所有转移性乳腺癌患者的60%~75%。骨转移灶形成的软组织包块可对周围重要神经血管形成压迫,导致肢体局部功能丧失,骨痛、病理性骨折、脊髓压迫及高钙血症等骨相关事件的出现,严重影响患者的自主活动能力及生存质量。为早期识别乳腺癌骨转移、控制骨转移灶进展并对骨转移灶及时进行干预,从而改善患者的生存质量,中国抗癌协会骨肿瘤和骨转移瘤专业委员会组织编写《乳腺癌骨转移诊疗专家共识》,以期对乳腺癌骨转移患者的诊疗给予指导与帮助。Abstract: The skeletal system is a common site for distant metastasis in patients with breast cancer, which accounts approximately 60%-75% of all metastatic breast cancers. Compression induced by the metastatic lesions on the neurovascular bundle often leads to a severe loss of the sensory and motor functions in the afflicted limb, including skeletal-related events (SREs), such as bone pain, pathologic fractures, and hypercalcemia spinal cord compression. The quality of life (QOL) of the patients is severely affected. Early diagnosis and timely intervention improve the QOL of patients with breast cancer and bone metastasis and reduce cancer-related mortality. The Bone Tumor and Bone Metastasis Committee of Chinese Anti-Cancer Association proposed this study titled “Expert consensus on the diagnosis and treatment of bone metastasis in patients with breast cancer,” as a clinical reference for the orthopedic oncologists.
-
Key words:
- metastatic breast cancer /
- bone metastasis /
- consensus
-
表 1 Mirels评分
变量 评分(分) 1 2 3 部位 上肢 下肢 转子周围 疼痛 轻度 中度 重度 病变性质 成骨性 混合 溶骨性 病变大小 <1/3 1/3-2/3 >2/3 ≤7分:病理骨折风险较低(<4%);8分:骨折风险为15%;9分:骨折风险达到33%;≥8分:应进行预防性内固定 表 2 脊柱骨转移不稳定性评估评分[39]
SINS组成 评分(分) SINS组成 评分(分) 部位 脊柱力线的放射学 结合部位(枕骨-C2,C7-T2,T11-L1,L5-S1) 3 半脱位 4 移动椎(C3-C6,L2-L4) 2 脊柱后凸,侧弯 2 半固定椎(T3-T10) 1 正常 0 固定椎(S2-S5) 0 椎体压缩骨折程度 疼痛 ≥50% 3 有 3 <50% 2 偶尔有,但不是活动痛 1 无塌陷但椎体侵犯>50% 1 无 0 无 0 骨病变性质 脊柱后柱受累情况 溶骨型 2 双侧 3 混合型 1 单侧 1 成骨型 0 无 0 0~6分稳定;>6分建议咨询骨科医师(7~12分潜在不稳定,13~18分不稳定) -
[1] Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015[J]. CA: A Cancer Journal for Clinicians, 2016, 66(2):115-132. [2] Harbeck N, Penault-Llorca F, Cortes J, et al. Breast cancer[J]. Breast cancer, Nat Rev Dis Primers, 2019, 5(1):66. [3] 郑荣寿, 孙可欣, 张思维,等.2015年中国恶性肿瘤流行情况分析,中华肿瘤杂志,2019,41(1):19-28. [4] Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity[J]. Clin Cancer Res, 2006, 12(20):6243s-6249s. [5] Hamaoka T, Madewell JE, Podoloff DA, et al. Bone imaging in metastatic breast cancer[J]. J Clin Oncol, 2004, 22(14):2942-2953. [6] Aurilio G, Disalvatore D, Pruneri G, et al. A meta-analysis of oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 discordance between primary breast cancer and metastases[J]. Eur J Cancer, 2014, 50(2):277-289. [7] Zulauf N, Brüggmann D, Groneberg D, et al. Expressiveness of bone markers in breast cancer with bone metastases[J]. Oncology, 2019, 97(4):236-244.. [8] 赵志庆,叶志鹏,燕太强,等.骨转移瘤患者生活质量评估的研究进展[J].中华骨科杂志,2017,37(18):1177-1184. [9] Gradishar WJ, Anderson BO, Abraham J, et al. Breast cancer, version 3.2020, NCCN clinical practice guidelines in oncology[J]. J Natl Compr Canc Netw, 2020, 18(4):452-478. [10] Swain SM, Miles D, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA):end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study[J]. The Lancet Oncology, 2020, 21(4):519-530. [11] Yan M, Bian L, Hu XC, et al. Pyrotinib plus capecitabine for human epidermal growth factor receptor 2-positive metastatic breast cancer after trastuzumab and taxanes (PHENIX): a randomized, double-blind, placebo-controlled phase 3 study[J]. Transl Breast Cancer Res, 2020, 1:13. [12] Barrett-Lee P, Casbard A, Abraham J, et al. Oral ibandronic acid versus intravenous zoledronic acid in treatment of bone metastases from breast cancer: a randomised, open label, non-inferiority phase 3 trial[J]. Lancet Oncol, 2014, 15(1):114-122. [13] Hortobagyi GN, van Poznak C, Harker WG, et al. Continued treatment effect of zoledronic acid dosing every 12 vs 4 weeks in women with breast cancer metastatic to bone[J]. JAMA Oncol, 2017, 3(7):906. [14] Zhang WJ, Bado I, Wang H, et al. Bone metastasis: find your niche and fit in[J]. Trends Cancer, 2019, 5(2):95-110. [15] Block GA, Bone HG, Fang L, et al. A single-dose study of denosumab in patients with various degrees of renal impairment[J]. J Bone Miner Res, 2012, 27(7):1471-1479. [16] Hanamura M, Iwamoto T, Soga N, et al. Risk factors contributing to the development of hypocalcemia after zoledronic acid administration in patients with bone metastases of solid tumor[J]. Biol Pharm Bull, 2010, 33(4):721-724.. [17] Bamias A, Kastritis E, Bamia C, et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors[J]. J Clin Oncol, 2005, 23(34):8580-8587. [18] Stopeck AT, Lipton A, Body JJ, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study[J]. J Clin Oncol, 2010, 28(35):5132-5139. [19] Gennari A, André F, Barrios CH, et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer[J]. Ann Oncol, 2021, 32(12):1475-1495. [20] Jabbari S, Gerszten PC, Ruschin M, et al. Stereotactic body radiotherapy for spinal metastases: practice guidelines, outcomes, and risks[J]. Cancer J, 2016, 22(4):280-289. [21] Lutz S, Berk L, Chang E, et al. Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline[J]. Int J Radiat Oncol, 2011, 79(4):965-976.. [22] Cox BW, Spratt DE, Lovelock M, et al. International Spine Radiosurgery Consortium consensus guidelines for target volume definition in spinal stereotactic radiosurgery[J]. Int J Radiat Oncol Biol Phys, 2012, 83(5):e597-e605. [23] Dunne EM, Fraser IM, Liu M. Stereotactic body radiation therapy for lung, spine and oligometastatic disease: current evidence and future directions[J]. Ann Transl Med, 2018, 6(14):283. [24] Zhuang HQ, Zhuang HX, Lang N, et al. Precision stereotactic radiotherapy for spinal tumors: mechanism, efficacy, and issues[J]. Front Oncol, 2020, 10:826. [25] Finlay IG, Mason MD, Shelley M. Radioisotopes for the palliation of metastatic bone cancer: a systematic review[J]. Lancet Oncol, 2005,6(6):392-400. [26] 中华医学会核医学分会转移性骨肿瘤治疗工作委员会.氯化锶[89Sr]治疗转移性骨肿瘤专家共识(2017年版),中华核医学与分子影像杂志,2018,38(6):412-415. [27] 郭卫.骨转移瘤的外科治疗策略[J].中国肺癌杂志,2009,12(6):42. [28] 郭卫.骨转移性肿瘤外科学[M].北京:人民卫生出版社,2013. [29] Forsberg JA, Eberhardt J, Boland PJ, et al. Estimating survival in patients with operable skeletal metastases: an application of a Bayesian belief network[J]. PLoS One, 2011, 6(5):e19956.. [30] Katagiri H, Okada R, Takagi T, et al. New prognostic factors and scoring system for patients with skeletal metastasis[J]. Cancer Med, 2014, 3(5):1359-1367. [31] Du W, Wang JC, Xu J, et al. Comparison and modification of survival predicting system for breast cancer patients with bone metastases[J]. Ann Joint, 2021, 6:28. [32] Tokuhashi Y, Uei H, Oshima M, et al. Scoring system for prediction of metastatic spine tumor prognosis[J]. World J Orthop, 2014, 5(3):262-271. [33] Tomita K, Kawahara N, Kobayashi T, et al. Surgical strategy for spinal metastases[J]. Spine, 2001, 26(3):298-306. [34] 郭卫,汤小东,杨毅,等.骨盆转移瘤外科治疗的疗效评估[J].中华外科杂志,2008,46(12):891-894. [35] 郭卫,姬涛.对脊柱转移癌如何进行合理的治疗[J].北京大学学报(医学版),2015,47(2):200-202. [36] 黄林,郭卫,杨荣利,等.远端肢体转移瘤的临床特点及外科治疗策略[J].中国骨与关节杂志,2014,3(7):490-496. [37] Mirels H. Metastatic disease in long bones A proposed scoring system for diagnosing impending pathologic fractures[J]. Clin Orthop Relat Res, 1989, 249(249):256-264. [38] Laufer I, Rubin DG, Lis E, et al. The NOMS framework: approach to the treatment of spinal metastatic tumors[J]. Oncologist, 2013, 18(6):744-751. [39] Fisher CG, DiPaola CP, Ryken TC, et al. A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine Oncology Study Group[J]. Spine, 2010, 35(22):E1221-E1229. [40] Manabe J, Kawaguchi N, Matsumoto S, et al. Surgical treatment of bone metastasis: indications and outcomes[J]. Int J Clin Oncol, 2005, 10(2):103-111. [41] Nazario J, Tam AL. Ablation of bone metastases[J]. Surg Oncol Clin N Am, 2011, 20(2):355-368. [42] 燕太强,郭卫.脊柱转移瘤的微创外科治疗进展[J].中国脊柱脊髓杂志,2011,21(3):244-247. [43] 郭卫,姬涛,杨毅,等.骨盆转移瘤外科治疗的方法及疗效分析[J].中华骨与关节外科杂志,2015,8(1):49-55. [44] 郭卫,孙馨,姬涛,等.髋臼转移瘤的外科治疗[J].中华外科杂志,2009,47(22):1718-1721. [45] Yang R, Goch A, Murphy D, et al. A novel tripod percutaneous reconstruction technique in periacetabular lesions caused by metastatic cancer[J]. J Bone Joint Surg Am, 2020, 102(7):592-599. [46] Harrington KD. The management of acetabular insufficiency secondary to metastatic malignant disease[J]. J Bone Joint Surg Am, 1981, 63(4):653-664. [47] 中华人民共和国国家卫生健康委员会, 癌症疼痛诊疗规范(2018年版)[J].临床肿瘤学杂志,2018,23(10):937-944. -



 下载:
下载:




