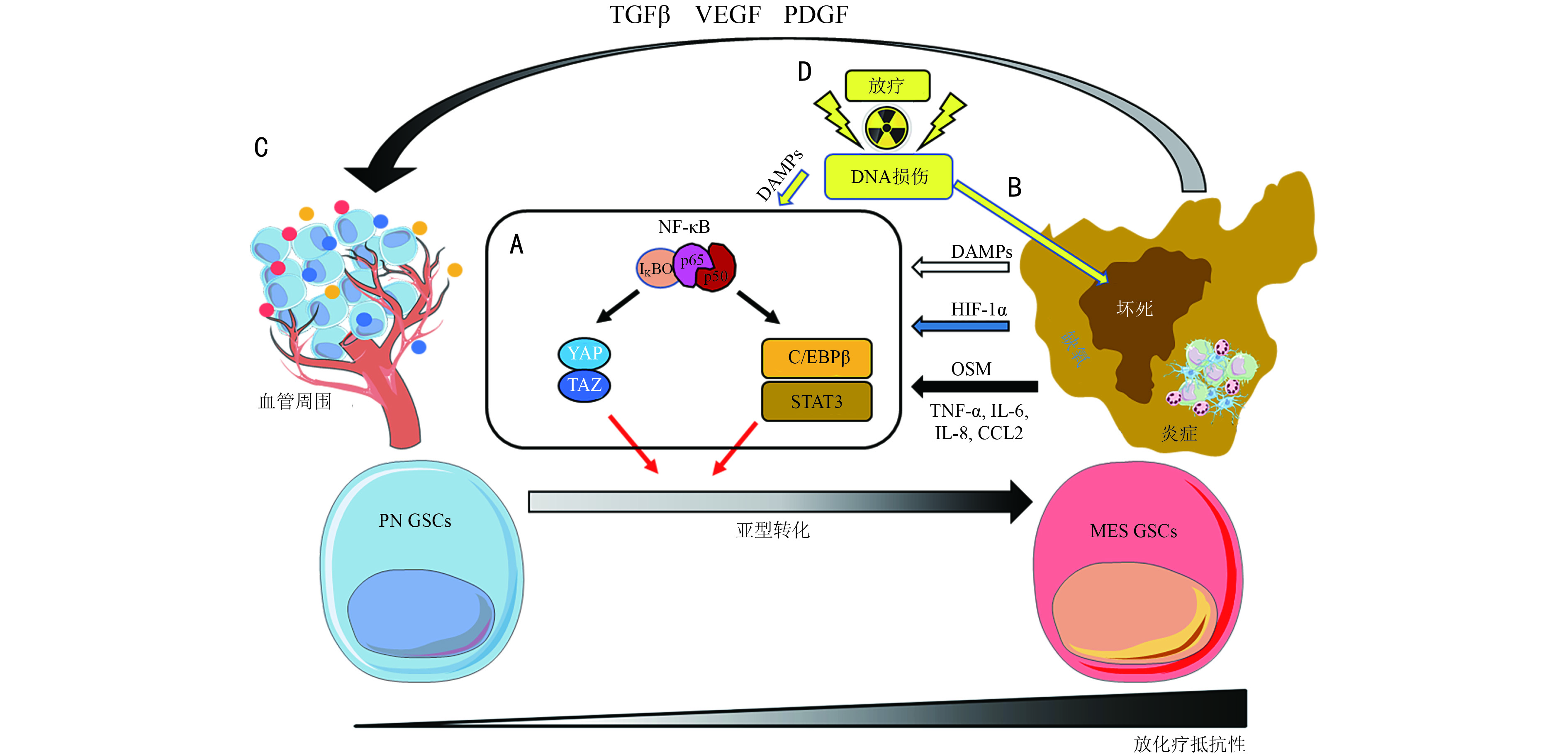
|
[1]
|
Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary[J]. Neuro Oncol, 2021, 23(8):1231-1251.
|
|
[2]
|
Teo WY, Sekar K, Seshachalam P, et al. Relevance of a TCGA-derived glioblastoma subtype gene-classifier among patient populations[J]. Sci Rep, 2019, 9(1):7442.
|
|
[3]
|
Guan XW, Vengoechea J, Zheng SY, et al. Molecular subtypes of glioblastoma are relevant to lower grade glioma[J]. PLoS One, 2014, 9(3):e91216.
|
|
[4]
|
Wang QH, Hu BL, Hu X, et al. Tumor evolution of glioma-intrinsic gene expression subtypes associates with immunological changes in the microenvironment[J]. Cancer Cell, 2018, 33(1):152.
|
|
[5]
|
van den Bent MJ, Gao Y, Kerkhof M, et al. Changes in the EGFR amplification and EGFRvⅢ expression between paired primary and recurrent glioblastomas[J]. Neuro Oncol, 2015, 17(7):935-941.
|
|
[6]
|
Wang JG, Cazzato E, Ladewig E, et al. Clonal evolution of glioblastoma under therapy[J]. Nat Genet, 2016, 48(7):768-776.
|
|
[7]
|
Hernández Martínez A, Madurga R, García-Romero N, et al. Unravelling glioblastoma heterogeneity by means of single-cell RNA sequencing[J]. Cancer Lett, 2022, 527:66-79.
|
|
[8]
|
Wang L, Babikir H, Müller S, et al. The phenotypes of proliferating glioblastoma cells reside on a single Axis of variation[J]. Cancer Discov, 2019, 9(12):1708-1719.
|
|
[9]
|
Patel AP, Tirosh I, Trombetta JJ, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma[J]. Science, 2014, 344(6190):1396-1401.
|
|
[10]
|
Bhat KPL, Balasubramaniyan V, Vaillant B, et al. Mesenchymal differentiation mediated by NF-κB promotes radiation resistance in glioblastoma[J]. Cancer Cell, 2013, 24(3):331-346.
|
|
[11]
|
Fedele M, Cerchia L, Pegoraro S, et al. Proneural-mesenchymal transition: phenotypic plasticity to acquire multitherapy resistance in glioblastoma[J]. Int J Mol Sci, 2019, 20(11):E2746.
|
|
[12]
|
Carro MS, Lim WK, Alvarez MJ, et al. The transcriptional network for mesenchymal transformation of brain tumours[J]. Nature, 2010, 463(7279):318-325.
|
|
[13]
|
Bhat KP, Salazar KL, Balasubramaniyan V, et al. The transcriptional coactivator TAZ regulates mesenchymal differentiation in malignant glioma[J]. Genes Dev, 2011, 25(24):2594-2609.
|
|
[14]
|
Yamini B. NF-κB, mesenchymal differentiation and glioblastoma[J]. Cells, 2018, 7(9):E125.
|
|
[15]
|
Jin X, Kim LJY, Wu QL, et al. Targeting glioma stem cells through combined BMI1 and EZH2 inhibition[J]. Nat Med, 2017, 23(11):1352-1361.
|
|
[16]
|
Markwell SM, Ross JL, Olson CL, et al. Necrotic reshaping of the glioma microenvironment drives disease progression[J]. Acta Neuropathol, 2022, 143(3):291-310.
|
|
[17]
|
Guan F, Jiang WF, Bai Y, et al. Purinergic P2X7 receptor mediates the elimination of Trichinella spiralis by activating NF-κB/NLRP3/IL-1β pathway in macrophages[J]. Infect Immun, 2021, 59(5):e00683-e00620.
|
|
[18]
|
Zanoni M, Sarti AC, Zamagni A, et al. Irradiation causes senescence, ATP release, and P2X7 receptor isoform switch in glioblastoma[J]. Cell Death Dis, 2022, 13(1):80.
|
|
[19]
|
Uribe D, Niechi I, Rackov G, et al. Adapt to persist: glioblastoma microenvironment and epigenetic regulation on cell plasticity[J]. Biology (Basel), 2022, 11(2):313.
|
|
[20]
|
Wang ZL, Shi YP, Ying CT, et al. Hypoxia-induced PLOD1 overexpression contributes to the malignant phenotype of glioblastoma via NF-κB signaling[J]. Oncogene, 2021, 40(8):1458-1475.
|
|
[21]
|
Okuyama Y, Tanaka Y, Jiang JJ, et al. Bmi1 regulates IκBα degradation via association with the SCF complex[J]. J Immunol, 2018, 201(8):2264-2272.
|
|
[22]
|
Kim Y, Varn FS, Park SH, et al. Perspective of mesenchymal transformation in glioblastoma[J]. Acta Neuropathol Commun, 2021, 9(1):50.
|
|
[23]
|
Schmitt MJ, Company C, Dramaretska Y, et al. Phenotypic mapping of pathologic cross-talk between glioblastoma and innate immune cells by synthetic genetic tracing[J]. Cancer Discov, 2021, 11(3):754-777.
|
|
[24]
|
Hara T, Chanoch-Myers R, Mathewson ND, et al. Interactions between cancer cells and immune cells drive transitions to mesenchymal-like states in glioblastoma[J]. Cancer Cell, 2021, 39(6):779-792.
|
|
[25]
|
Niklasson M, Bergström T, Jarvius M, et al. Mesenchymal transition and increased therapy resistance of glioblastoma cells is related to astrocyte reactivity[J]. J Pathol, 2019, 249(3):295-307.
|
|
[26]
|
Pan YB, Wang SQ, Yang B, et al. Transcriptome analyses reveal molecular mechanisms underlying phenotypic differences among transcriptional subtypes of glioblastoma[J]. J Cell Mol Med, 2020, 24(7):3901-3916.
|
|
[27]
|
Brandes AA, Gil-Gil M, Saran F, et al. A randomized phase II trial (TAMIGA) evaluating the efficacy and safety of continuous bevacizumab through multiple lines of treatment for recurrent glioblastoma[J]. Oncologist, 2019, 24(4):521-528.
|
|
[28]
|
Chandra A, Jahangiri A, Chen W, et al. Clonal ZEB1-driven mesenchymal transition promotes targetable oncologic antiangiogenic therapy resistance[J]. Cancer Res, 2020, 80(7):1498-1511.
|
|
[29]
|
Minata M, Audia A, Shi JF, et al. Phenotypic plasticity of invasive edge glioma stem-like cells in response to ionizing radiation[J]. Cell Rep, 2019, 26(7):1893-1905.
|
|
[30]
|
Zhai K, Huang Z, Huang Q, et al. Pharmacological inhibition of BACE1 suppresses glioblastoma growth by stimulating macrophage phagocytosis of tumor cells[J]. Nat Cancer, 2021, 2(11):1136-1151.
|




 下载:
下载: