Efficacy and safety of blinatumomab in the treatment of B-cell acute lymphoblastic leukemia: a retrospective study
-
摘要:
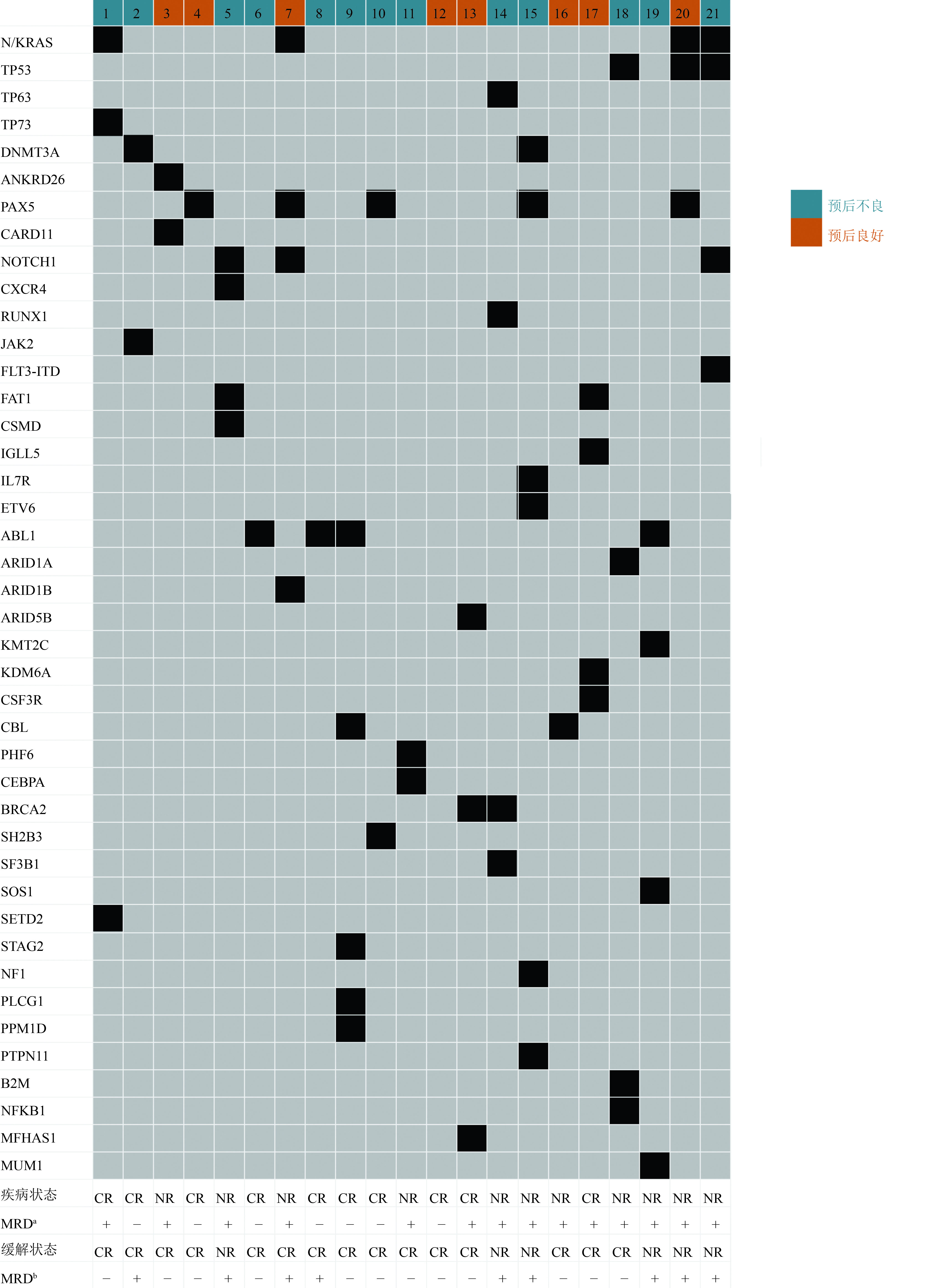
目的 评估博纳吐单抗在急性B淋巴细胞白血病(B-cell acute lymphoblastic leukemia,B-ALL)患者治疗中的近期疗效和安全性。 方法 回顾性分析2021年8月至2022年4月于苏州大学附属第一医院及苏州弘慈血液病医院接受博纳吐单抗治疗的21例B-ALL患者的临床及随访资料。 结果 21例B-ALL患者,中位随访5.1(1.3~8.3)个月。11例复发/难治性(relapsed and refractory,R/R)患者中,45.5%获得完全缓解(complete response,CR)/CR伴血液学不完全恢复(CR with incomplete hematological recovery,CRi),其中80.0%达到微小残留病(minimal residual disease,MRD)阴性。3例CR/CRi且MRD阳性者全部出现MRD反应。14例MRD阳性患者中,5例桥接异基因造血干细胞移植,9例未移植,6个月总生存率(overall survival,OS)分别为100%和76.0%(P=0.260),2个月无白血病生存率(leukemia-free survival,LFS)分别为80.0%和33.0%(P=0.044)。既往化疗次数少于3次者较3次及以上者拥有更长久的LFS(P=0.001)。所有患者均出现任何等级的不良事件,仅1例出现3级细胞因子释放综合征,未出现致命性不良事件。 结论 博纳吐单抗在R/R和MRD阳性B-ALL患者中均获得较高的治疗反应率,早期应用有利于获得深层缓解后尽早桥接移植,延长无病生存期。 -
关键词:
- 博纳吐单抗 /
- 双特异性抗体 /
- 急性B淋巴细胞白血病 /
- 有效性 /
- 安全性
Abstract:Objective To evaluate the short-term efficacy and safety of blinatumomab in the treatment of patients with B-cell acute lymphoblastic leukemia (B-ALL). Methods Clinical and follow-up data for 21 patients with B-ALL who received blinatumomab from August 2021 to April 2022 at The First Affiliated Hospital of Soochow University and Soochow Hopes Hematonosis Hospital were retrospectively analyzed. Results All 21 patients were followed up regularly with a median follow-up time of 5.1 (1.3–8.3) months. Of the 11 patients with relapsed or refractory (R/R) B-ALL, the complete response (CR) or CR with incomplete hematological recovery (CRi) rate was 45.5% and the minimal residual disease (MRD)-negative rate among patients with a CR or a CRi was 80.0%. All three patients with a MRD-positive CR or CRi achieved MRD negativity. Of the 14 MRD-positive patients, five patients received allogeneic hematopoietic stem cell transplantation (allo-HSCT) after blinatumomab treatment, and nine patients did not. The 6-month overall survival rates of the allo-HCST and non-allo-HCST groups were 100.0% and 76.0% (P=0.260), and the 2-month leukemia-free survival (LFS) rates were 80.0% and 33.0% (P=0.044), respectively. In addition, patients who received less than three times of chemotherapy had longer LFS than those who received three or more times of chemotherapy (P=0.001). All the patients experienced adverse events of any grade. Only one patient experienced grade 3 of cytokine release syndrome, but no fatal adverse events occurred. Conclusions Blinatumomab achieved high treatment response rates in both R/R and MRD-positive B-ALL patients, which is conducive to early bridging transplantation after deep remission and prolonging leukemia free survival. -
Key words:
- blinatumomab /
- bispecific antibody /
- B-cell acute lymphoblastic leukemia (ALL) /
- efficacy /
- safety
-
表 1 21例接受博纳吐单抗治疗B-ALL患者的临床特征
特征 例数(%) 特征 例数(%) 性别 既往化疗次数(次) 男 10(47.6) <3 11(52.4) 女 11(52.4) ≥3 10(47.6) 分型(型) 既往移植 5(23.8) B-I 3(14.3) 既往CAR-T治疗 4(19.0) B-II 17(81.0) 复发次数(次) B-III 1(4.8) 1 10(47.6) 合并中枢神经系统白血病 1(4.8) ≥2 2(9.5) 特殊细胞遗传学标志 复发类型a Ph样或BCR-ABL1样 6(28.6) 血液学复发 1(4.8) t(v;14q32)/IgH 5(23.8) 分子学复发 2(9.5) Ph或BCR-ABL1 3(14.3) 髓外复发 1(4.8) MLL/AF4 1(4.8) 血液学和髓外复发 1(4.8) 低二倍体 1(4.8) 预后分层 良好 8(38.1) 不良 13(61.9) a:博纳吐单抗治疗后复发类型 表 2 接受博纳吐单抗治疗中出现的不良事件
例(%) 不良反应 任何级别 ≥3级 血液学不良事件 白细胞减少 21(100.0) 14(66.7) 中性粒细胞计数减少 21(100.0) 15(71.4) 血小板计数减少 16(76.2) 13(61.9) 贫血 21(100.0) 12(57.1) 非血液学不良事件 细胞因子释放综合征 12(57.1) 1(4.8) 神经系统症状 5(23.8) 0(0) 低钾血症 9(42.9) 0(0) 高血压 2(9.5) 1(4.8) 恶心等胃肠道不适 2(9.5) 0(0) 肝功能损伤 12(57.1) 0(0) 肾功能损伤 1(4.8) 0(0) 纤维蛋白原降低 2(9.5) 0(0) APTT延长 12(57.1) 0(0) 皮肤丘疹 1(4.8) 0(0) 全身乏力 2(9.5) 0(0) 骨痛 1(4.8) 0(0) 结膜炎 1(4.8) 0(0) -
[1] El Chaer F, Holtzman NG, Sausville EA, et al. Relapsed philadelphia chromosome-positive Pre-B-ALL after CD19-directed CAR-T cell therapy successfully treated with combination of blinatumomab and ponatinib[J]. Acta Haematol, 2019, 141(2):107-110. doi: 10.1159/000495558 [2] 中国抗癌协会血液肿瘤专业委员会,中华医学会血液学分会白血病淋巴瘤学组.中国成人急性淋巴细胞白血病诊断与治疗指南(2021年版)[J].中华血液学杂志,2021,42(9):705-716. doi: 10.3760/cma.j.issn.0253-2727.2021.09.001 [3] Li B, Brady SW, Ma X, et al. Therapy-induced mutations drive the genomic landscape of relapsed acute lymphoblastic leukemia[J]. Blood, 2020, 135(1):41-55. doi: 10.1182/blood.2019002220 [4] Hathaway L, Sen JM, Keng M. Impact of blinatumomab on patient outcomes in relapsed/refractory acute lymphoblastic leukemia: evidence to date[J]. Patient Relat Outcome Meas, 2018, 9:329-337. doi: 10.2147/PROM.S149420 [5] Gaballa MR, Banerjee P, Milton DR, et al. Blinatumomab maintenance after allogeneic hematopoietic cell transplantation for B-lineage acute lymphoblastic leukemia[J]. Blood, 2022, 139(12):1908-1919. doi: 10.1182/blood.2021013290 [6] Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia[J]. Blood, 2016, 127(20):2391-2405. doi: 10.1182/blood-2016-03-643544 [7] Brown PA, Shah B, Advani A, et al. Acute lymphoblastic leukemia, version 2.2021, NCCN clinical practice guidelines in oncology[J]. J Natl Compr Canc Netw, 2021, 19(9):1079-1109. doi: 10.6004/jnccn.2021.0042 [8] Topp MS, Gökbuget N, Stein AS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study[J]. Lancet Oncol, 2015, 16(1):57-66. doi: 10.1016/S1470-2045(14)71170-2 [9] Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia[J]. N Engl J Med, 2017, 376(9):836-847. doi: 10.1056/NEJMoa1609783 [10] Gökbuget N, Dombret H, Bonifacio M, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia[J]. Blood, 2018, 131(14):1522-1531. doi: 10.1182/blood-2017-08-798322 [11] Gökbuget N, Zugmaier G, Dombret H, et al. Curative outcomes following blinatumomab in adults with minimal residual disease B-cell precursor acute lymphoblastic leukemia[J]. Leuk Lymphoma, 2020, 61(11):2665-2673. doi: 10.1080/10428194.2020.1780583 [12] Brown PA, Ji L, Xu X, et al. Effect of postreinduction therapy consolidation with blinatumomab vs. chemotherapy on disease-free survival in children, adolescents, and young adults with first relapse of B-cell acute lymphoblastic leukemia: a randomized clinical trial[J]. Jama, 2021, 325(9):833-842. doi: 10.1001/jama.2021.0669 [13] Dombret H, Topp MS, Schuh AC, et al. Blinatumomab versus chemotherapy in first salvage or in later salvage for B-cell precursor acute lymphoblastic leukemia[J]. Leuk Lymphoma, 2019, 60(9):2214-2222. doi: 10.1080/10428194.2019.1576872 [14] Aldoss I, Yang D, Malki MMA, et al. Allogeneic hematopoietic cell transplantation for relapsed and refractory philadelphia negative B cell aLL in the Era of novel salvage therapies[J]. Transplant Cell Ther, 2021, 27(3):e251-255. [15] Contreras CF, Higham CS, Behnert A, et al. Clinical utilization of blinatumomab and inotuzumab immunotherapy in children with relapsed or refractory B-acute lymphoblastic leukemia[J]. Pediatr Blood Cancer, 2021, 68(1):e28718. -




 下载:
下载: