Efficacy and safety of BTK inhibitors in the treatment of central nervous system lymphoma
-
摘要:
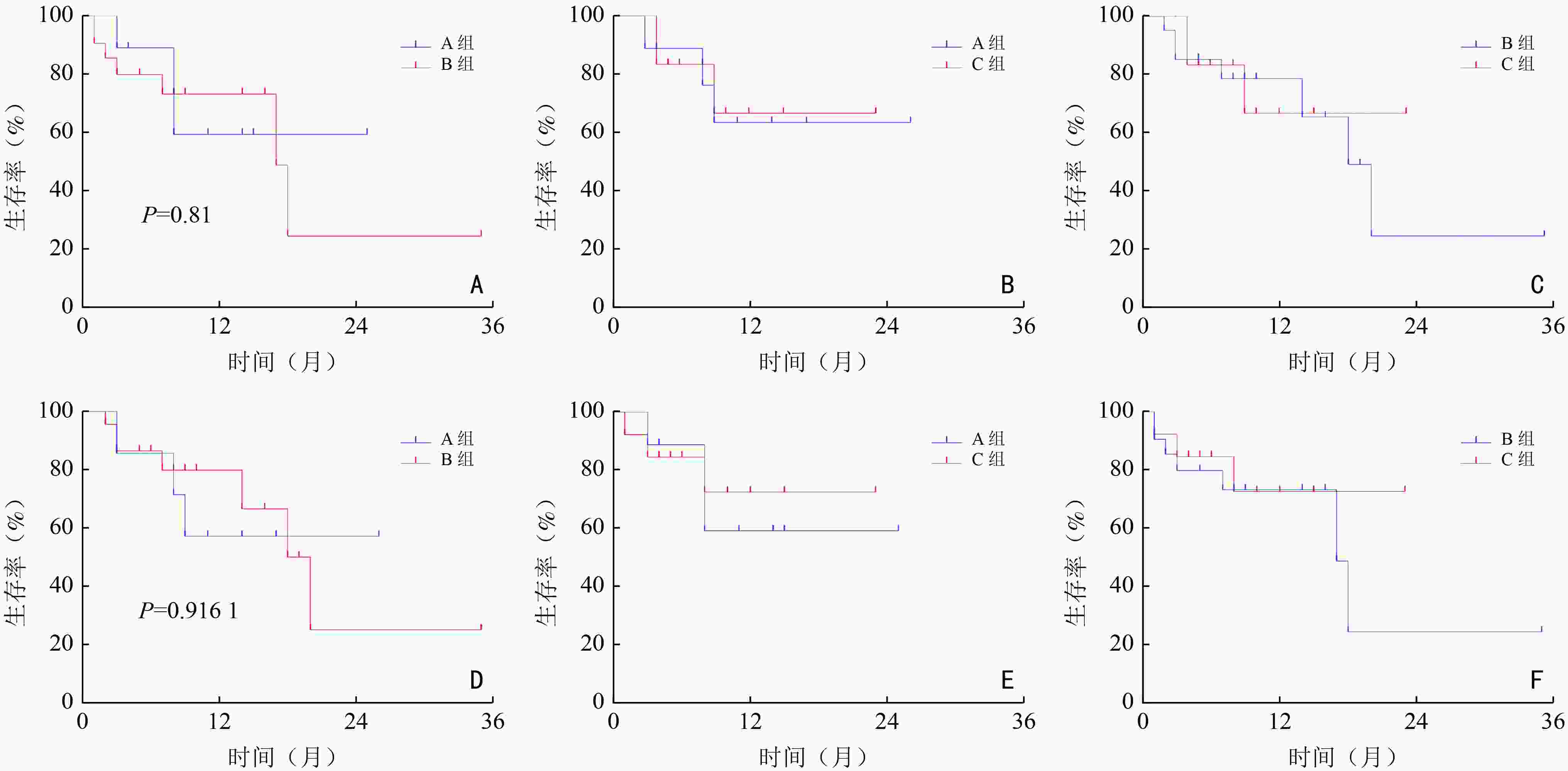
目的 研究布鲁顿酪氨酸激酶(Bruton's tyrosine kinase,BTK)抑制剂治疗复发/难治性中枢神经系统淋巴瘤(relapsed/refractory central nervous system lymphoma,R/R CNSL)的有效性和安全性。 方法 回顾性分析郑州大学第一附属医院2018年10月至2021年10月收治的43例R/R CNSL患者,分别使用BTK抑制剂单药、BTK抑制剂联合化疗、BTK抑制剂联合免疫治疗方案,BTK抑制剂包括伊布替尼、泽布替尼、奥布替尼,初始剂量分别为(420~560)mg/d、320 mg/d、(100~150)mg/d,分析治疗后的最好疗效和不良反应。 结果 BTK抑制剂单药组(A组)、BTK抑制剂联合化疗组(B组)、BTK抑制剂联合免疫治疗组(C组)的客观缓解率(objective response rate,ORR)分别为44.4%、85.7%和76.9%,B组的ORR高于A、C组,原发中枢神经系统淋巴瘤(primary CNSL,PCNSL)患者的ORR(78.6% vs. 66.7%)高于继发中枢神经系统淋巴瘤(secondary CNSL,SCNSL)患者,两者的无进展生存期(progression-free survival,PFS)和总生存期(overall survival,OS)差异均无统计学意义(均P>0.05)。3/4级不良反应主要为白细胞减少、血小板减少、贫血。贫血。 结论 BTK抑制剂单药及联合方案对R/R CNSL患者疗效可,不良反应可耐受。 Abstract:Objective To investigate the efficacy and safety of Bruton's tyrosine kinase (BTK) inhibitors in the treatment of relapsed/refractory central nervous system lymphoma (R/R CNSL). Methods A retrospective analysis of 43 patients with R/R CNSL admitted to The First Affiliated Hospital of Zhengzhou University from October 2018 to October 2021, who were treated with BTK inhibitor monotherapy (group A), BTK inhibitor combined with chemotherapy (group B), or BTK inhibitor combined with immunotherapy regimens (group C) was conducted. BTK inhibitors included ibrutinib, zanubrutinib, and obrutinib, with initial doses of (420–560) mg/day, 320 mg/day, and (100–150) mg/day, respectively. After treatment, the efficacy and adverse reactions were analyzed. Results The objective response rate (ORR) of groups A, B, and C were 44.4%, 85.7%, and 76.9%, respectively. The ORR of group B was higher than that of groups A and C. Patients with primary central nervous system lymphoma (PCNSL) had a higher ORR (78.6% vs. 66.7%) than patients with secondary central nervous system lymphoma (SCNSL). Progression-free survival (PFS) (P=0.67) and overall survival (OS) (P=0.77) were not significantly different between patients with PCNSL and SCNSL. Grade 3/4 adverse reactions were mainly leukopenia, thrombocytopenia, and anemia. Conclusions The efficacy of BTK inhibitors as single drugs and combined regimens for patients with R/R CNSL is acceptable, and the adverse reactions are tolerable. -
表 1 43例R/R CNSL患者的治疗方案
患者特征 例(%) BTK抑制剂单药组(A组) 9(21.0) 伊布替尼 6(14.0) 泽布替尼 1(2.3) 奥布替尼 2(4.7) BTK抑制剂联合化疗组(B组) 21(48.8) BTK抑制剂+R/FPD/R-FPD 16(37.2) BTK抑制剂+MT 1(2.3) BTK抑制剂+R-M 1(2.3) 其他 3(7.0) BTK抑制剂联合免疫治疗组(C组) 13(30.2) BTK抑制剂+来那度胺/沙利度胺 9(20.9) BTK抑制剂+PD-1+来那度胺 3(7.0) BTK抑制剂+PD-1 1(2.3) R:利妥昔单抗;F:福莫司汀;P:培美曲塞;D:地塞米松;M:甲氨蝶呤;T:替莫唑胺;PD-1:卡瑞利珠单抗 表 2 患者的基本资料和特征
患者特征 例(%) 性别 男性 22(51.2) 女性 21(48.8) 脑脊液细胞学阳性 是 14(32.6) 否 29(67.4) 侵犯脑深部结构 是 21(48.8) 否 20(46.5) 未知 2(4.7) BTK抑制剂药物及剂量 伊布替尼(mg) 560 16(37.2) 420 1(2.3) 泽布替尼(mg) 320 3(7.0) 奥布替尼(mg) 150 22(51.2) 100 1(2.3) 既往一线治疗方案 (R)FPD 方案 17(39.5) FTD 方案 8(18.6) MTX联合方案 5(11.6) R-CHOP方案 12(27.9) PD-1联合方案 1(2.3) 表 3 A、B、C三组患者的疗效比较
反应率 A组(%) BTK抑制剂联合治疗组 P B组(%) C组(%) CR/CRu 0(0) 5(23.8) 3(23.1) 0.311 PR 4(44.4) 13(61.9) 7(53.8) 0.668 SD 3(33.3) 1(4.8) 1(7.7) 0.109 PD 2(22.2) 2(9.5) 2(15.4) 0.630 ORR 4(44.4) 18(85.7) 10(76.9) 0.070 DCR 7(77.8) 19(90.5) 11(84.6) 0.630 A组:BTK抑制剂单药组;B组:BTK抑制剂联合化疗组;C组:BTK抑剂联合免疫治疗组;CR/CRu:完全缓解/未确认的完全缓解;PR:部分缓解;SD:疾病稳定;PD:疾病进展;ORR:客观缓解率;DCR:疾病控制率 表 4 43例CNSL患者的不良反应发生情况
不良反应指标 A组 B组 C组 合计(%) 1/2 3/4 1/2 3/4 1/2 3/4 白细胞减少 3(33.3) 1(11.1) 8(38.1) 7(33.3) 4(30.8) 5(38.5) 28(65.1) 血小板减少 2(22.2) 0(0) 5(23.8) 5(23.8) 4(30.8) 2(15.4) 18(41.9) 贫血 2(22.2) 0(0) 12(57.1) 4(19.0) 5(38.5) 1(7.7) 24(55.8) 甲功异常 1(11.1) 0(0) 3(14.3) 0(0) 2(15.4) 0(0) 5(11.6) ALT/AST升高 1(11.1) 0(0) 2(9.5) 1(4.8) 0(0) 0(0) 4(9.3) 低钾血症 0(0) 0(0) 7(33.3) 2(9.5) 1(7.7) 1(7.7) 11(25.6) 高糖血症 0(0) 0(0) 4(19.0) 0(0) 3(23.1) 0(0) 7(16.3) 低蛋白血症 0(0) 0(0) 3(14.3) 0(0) 2(15.4) 0(0) 5(11.6) 高胆红素血症 1(11.1) 0(0) 2(9.5) 0(0) 0(0) 0(0) 3(7.0) 皮疹 1(11.1) 0(0) 2(9.5) 0(0) 1(7.7) 0(0) 4(9.3) 乏力 0(0) 0(0) 0(0) 0(0) 2(15.4) 1(7.7) 3(7.0) 肺部感染 0(0) 0(0) 0(0) 0(0) 1(7.7) 0(0) 1(2.3) A组:BTK抑制剂单药组;B组:BTK抑制剂联合化疗组;C组:BTK抑剂联合免疫治疗组;ALT:丙氨酸氨基转移酶;AST:天冬氨酸氨基转移酶 -
[1] Nayak L, Iwamoto FM, LaCasce A, et al. PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma[J]. Blood, 2017, 129(23):3071-3073. doi: 10.1182/blood-2017-01-764209 [2] Li Q, Liu W, Li K, et al. Diagnosis and individualized treatment of secondary central nervous system lymphoma: acase report[J]. Onco Targets Ther, 2021, 14:3167-3175. doi: 10.2147/OTT.S300805 [3] Chukwueke U, Grommes C, Nayak L. Primary central nervous system lymphomas[J]. Hematol Oncol Clin North Am, 2022, 36(1):147-159. doi: 10.1016/j.hoc.2021.09.004 [4] 高凤华,吴晶晶.伊布替尼治疗原发性中枢神经系统淋巴瘤的临床研究进展[J].癌症进展,2020,18(11):1081-1084. [5] Ren LL, Li L, Zhang L, et al. Ibrutinib in patients with relapsed or refractory diffuse large B-cell lymphoma: aretrospective study[J]. Indian J Hematol Blood Transfus, 2022, 38(1):42-50. doi: 10.1007/s12288-021-01433-w [6] Narita Y, Nagane M, Mishima K, et al. Phase Ⅰ/Ⅱ study of tirabrutinib, a second-generation Bruton's tyrosine kinase inhibitor, in relapsed/refractory primary central nervous system lymphoma[J]. Neuro Oncol, 2020, 23(1):122-133. [7] Abrey LE, Batchelor TT, FerreriAJM, et al. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma[J]. J Clin Oncol, 2005, 23(22):5034-5043. doi: 10.1200/JCO.2005.13.524 [8] Freites-Martinez A, Santana N, Arias-Santiago S, et al. Using the common terminology criteria for adverse events (CTCAE-version 5.0) to evaluate the severity of adverse events of anticancer therapies[J]. Actas Dermosifiliogr (Engl Ed), 2021, 112(1):90-92. [9] Correia CE, Schaff LR, Grommes C. Central nervous system lymphoma: approach to diagnosis and treatment[J]. Cancer J, 2020, 26(3):241-252. doi: 10.1097/PPO.0000000000000449 [10] Cheah CY, Joske D, Cull G, et al. High-dose therapy and autologous stem cell transplantation may only be applicable to selected patients with secondary CNS diffuse large B-cell lymphoma[J]. Br J Haematol, 2017, 178(6):991-994. doi: 10.1111/bjh.14187 [11] Soussain C, Choquet S, Blonski M, et al. Ibrutinib monotherapy for relapse or refractory primary CNS lymphoma and primary vitreoretinal lymphoma: final analysis of the phaseⅡ 'proof-of-concept' iLOC study by the lymphoma study association (LYSA) and the French oculo-cerebral lymphoma (LOC) network[J]. Eur J Cancer, 2019, 117:121-130. doi: 10.1016/j.ejca.2019.05.024 [12] Lionakis MS, Dunleavy K, Roschewski M, et al. Inhibition of B cell receptor signaling by ibrutinib in primary CNS lymphoma[J]. Cancer Cell, 2017, 31(6):833-843. doi: 10.1016/j.ccell.2017.04.012 [13] Lewis KL, Chin CK, Manos K, et al. Ibrutinib for central nervous system lymphoma: the australasian lymphoma alliance/MD Anderson cancer center experience[J]. Br J Haematol, 2021, 192(6):1049-1053. doi: 10.1111/bjh.16946 [14] Grommes C, Pastore A, Palaskas N, et al. Ibrutinib unmasks critical role of brutontyrosine kinase in primary CNS lymphoma[J]. Cancer Discov, 2017, 7(9):1018-1029. doi: 10.1158/2159-8290.CD-17-0613 [15] Grommes C, Tang SS, Wolfe J, et al. Phase 1b trial of an ibrutinib-based combination therapy in recurrent/refractory CNS lymphoma[J]. Blood, 2019, 133(5):436-445. doi: 10.1182/blood-2018-09-875732 [16] Guaitani A, Corada M, Lucas C, et al. Pharmacokinetics of fotemustine and BCNU in plasma, liver and tumor tissue of rats bearing two lines of Walker 256 carcinoma[J]. Cancer Chemother Pharmacol. 1991, 28(4): 293-297. [17] Wu J, Duan L, Zhang L, et al. Fotemustine, teniposide and dexamethasone versus high-dose methotrexate plus cytarabine in newly diagnosed primary CNS lymphoma: a randomised phase 2 trial[J]. J Neurooncol, 2018, 140(2):427-434. doi: 10.1007/s11060-018-2970-x [18] Wu J, Gao F, Wang W, et al. Fotemustine-based therapy in combination with rituximab as a first-line induction chemotherapy followed by WBRT for newly diagnosed primary central nervous system lymphoma: a prospective phaseⅡtrial[J]. Cancer Biol Med., 2021, 42(2):223-226. [19] Alcantara M, Fuentealba J, Soussain C. Emerging landscape of immunotherapy for primary central nervous system lymphoma[J]. Cancers (Basel), 2021, 13(20):5061. doi: 10.3390/cancers13205061 [20] Wang Y, Zhang LL, Champlin RE, et al. Targeting Bruton's tyrosine kinase with ibrutinib in B-cell malignancies[J]. Clin Pharmacol Ther, 2015, 97(5):455-468. [21] 庞露,陶丽,刘立民,等.伊布替尼致不良反应文献分析[J].中国医院药学杂志,2020,40(14):1573-1576. doi: 10.13286/j.1001-5213.2020.14.14 [22] 王京京,詹成创,苏梦琦,等.伊布替尼相关性心房颤动研究进展[J].国际心血管病杂志,2020,47(2):92-96. doi: 10.3969/j.issn.1673-6583.2020.02.008 [23] Gu D, Tang H, Wu J, et al. Targeting Bruton tyrosine kinase using non-covalent inhibitors in B cell malignancies[J]. J Hematol Oncol., 2021, 14(1):40. doi: 10.1186/s13045-021-01049-7 -



 下载:
下载: