-
摘要:
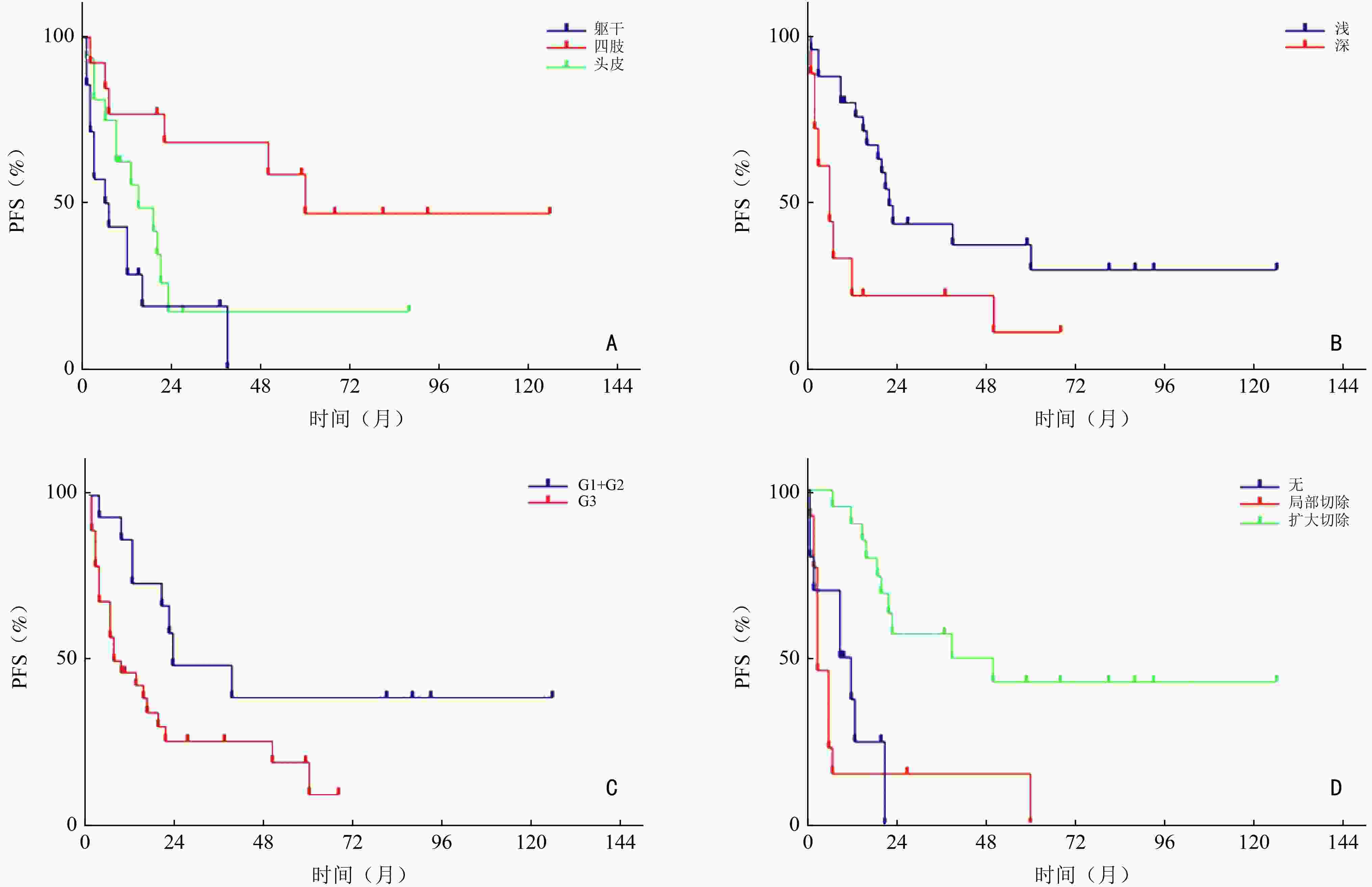
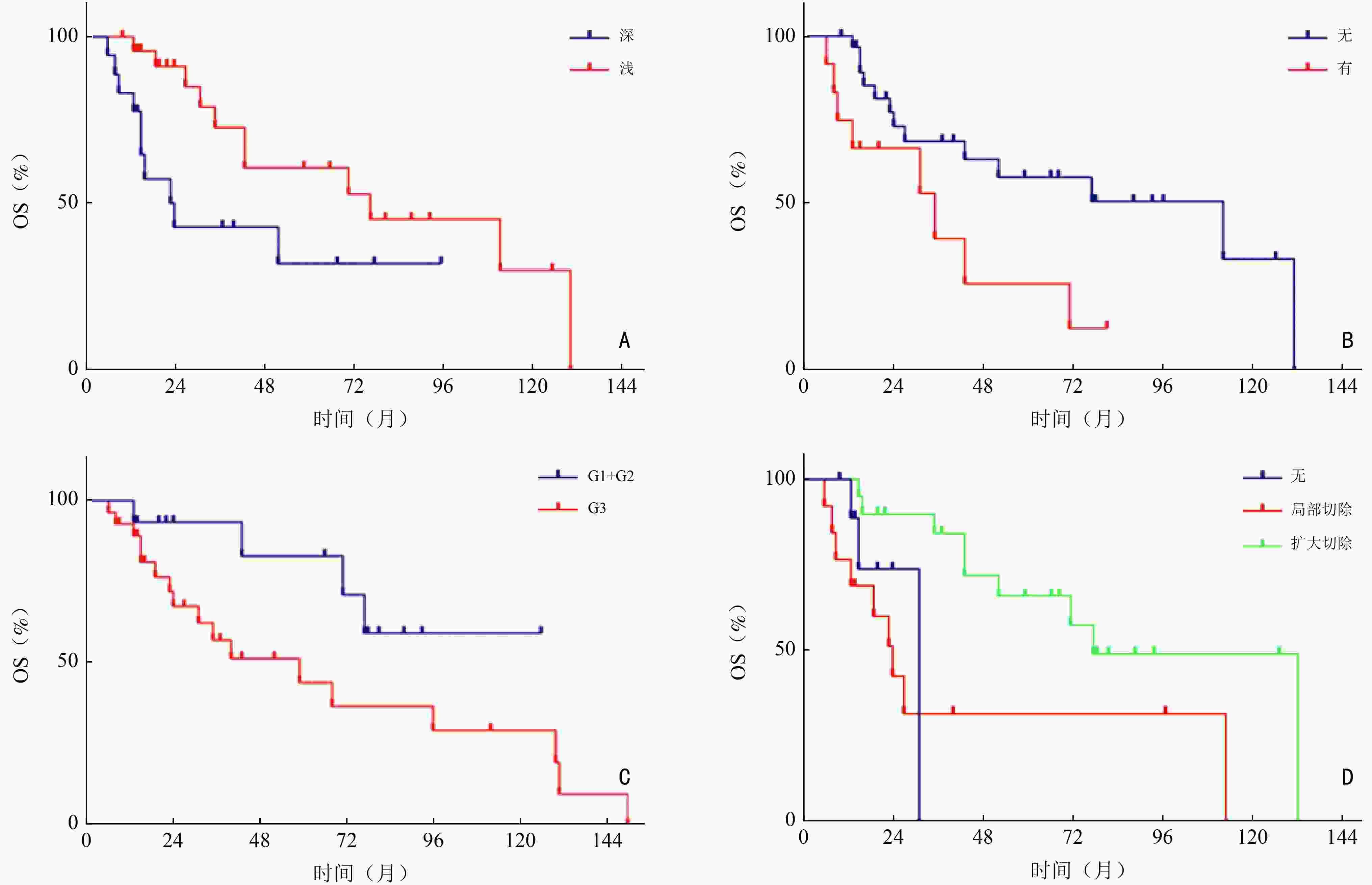
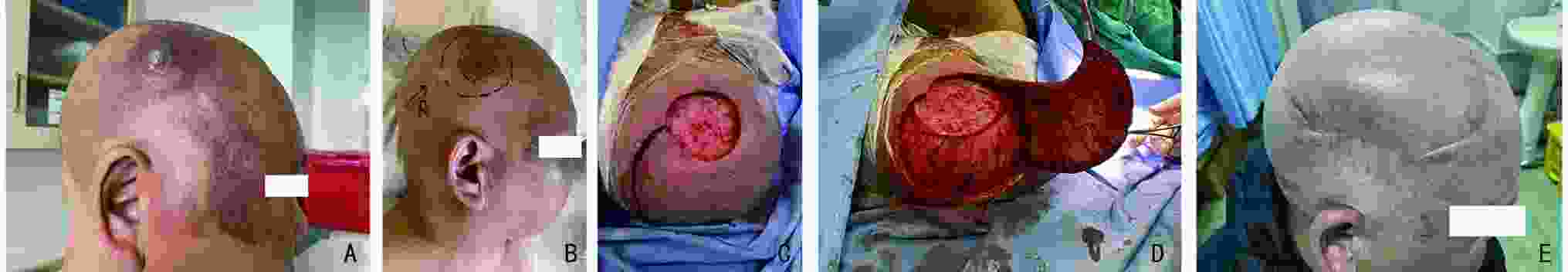
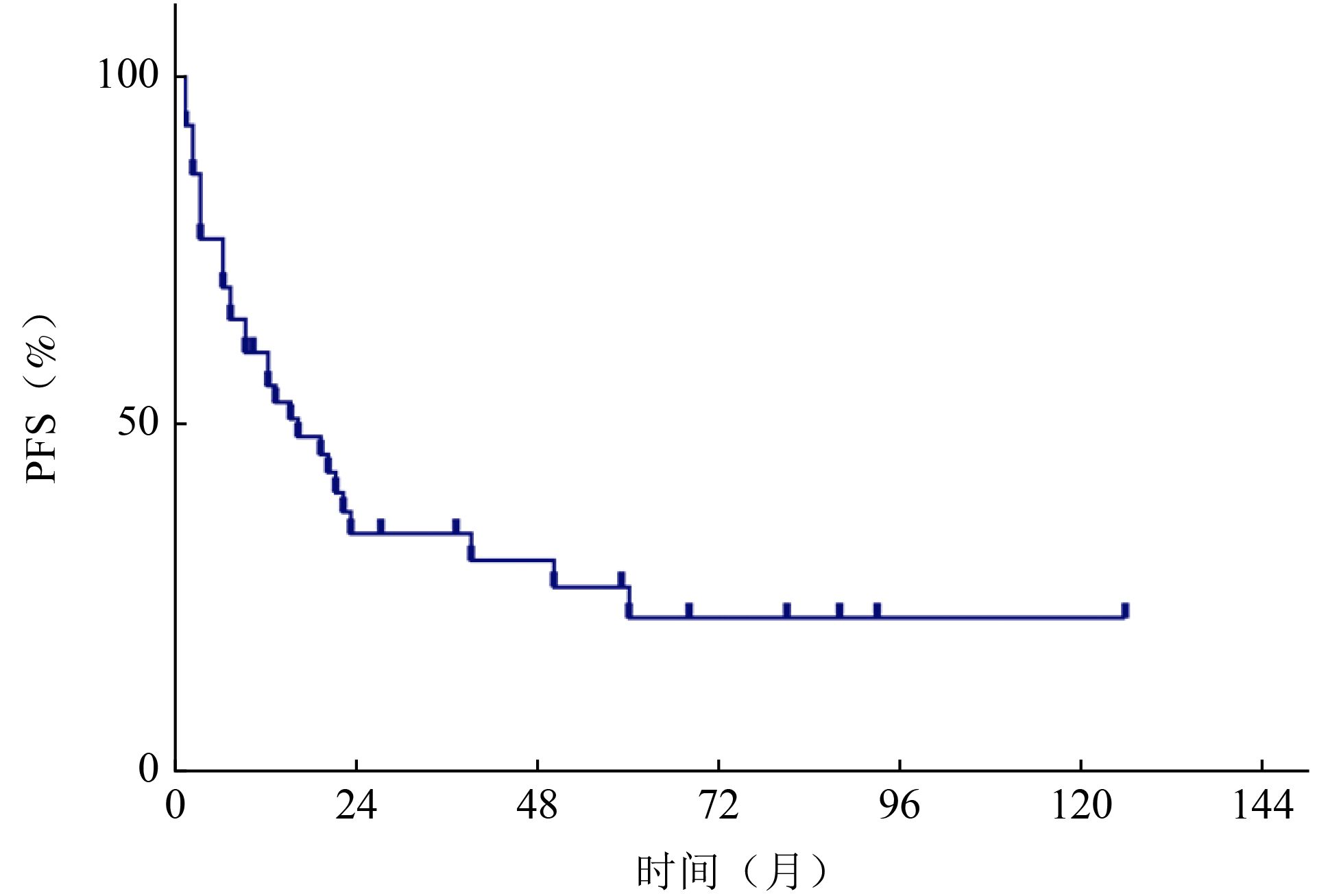
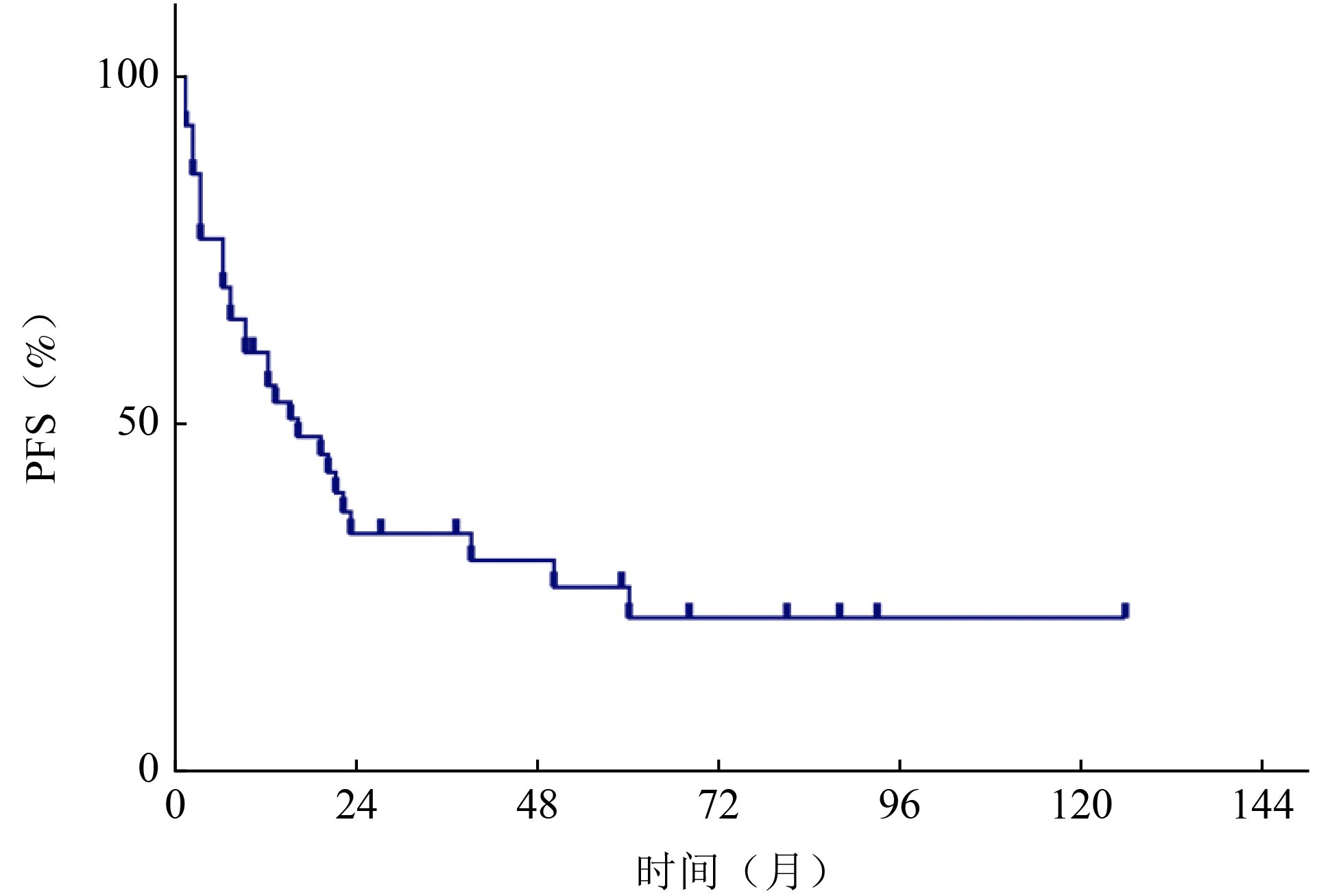
目的 回顾性分析血管肉瘤(angiosarcoma,AS)患者临床治疗结果,并进行分析比较,确定结果的预测因素。 方法 收集2000年1月至2021年3月北京大学肿瘤医院收治的43例血管肉瘤患者临床资料,采用SPSS 20.0软件进行统计学单因素及多因素分析,主要疗效指标包括无进展生存期(progression-free survival,PFS)和总生存期(overall survival,OS),随访方式包括住院、门诊及电话随访,截止时间为2022年5月。 结果 截至随访结束,43例患者中死亡21例(49%),生存22例(51%),中位随访时间为52(6~131) 个月。中位PFS为16个月,中位OS为52个月。1、3、5年的PFS分别为55.6%、34.3%和22.2%;OS为93%、61.1%和49.7%。单因素分析显示,发病部位、肿瘤深度、分级及治疗方式与PFS相关;肿瘤深度、破溃、分级及治疗方式与OS相关(P<0.05)。多因素分析显示,肿瘤深度及治疗方式是PFS的独立预后因素;肿瘤深度、破溃、分级是OS的独立预后因素(P<0.05)。 结论 血管肉瘤临床以手术治疗为基础,但要避免盲目非计划手术;患者或可以从新辅助治疗中获益,化疗以紫杉类和蒽环类为主,对于局部晚期的患者可进行术前转化治疗;放疗对提高局部控制率有帮助;应重视手术、化疗、放疗的综合治疗。 Abstract:Objective The clinical treatment results of 43 patients with angiosarcoma were retrospectively analyzed and compared to determine the predictive factors of the results, so as to help clinicians choose a more appropriate plan for patients. Methods The clinical data of patients with angiosarcoma admitted to Peking University Cancer Hospital & Institute from January 2000 to March 2021 were collected. SPSS 20.0 software was used for statistical univariate and multivariate analysis. The main efficacy indexes included progression-free survival (PFS) and overall survival (OS) , including inpatient, outpatient and telephone follow-up, until May 2022. Results During the follow-up period, 21 patients (49%) died, and 22 patients (51%) survived. The median follow-up time of the whole case was 52 months (range: 6-131 months), and the median PFS was 16 months, OS was 52 months, PFS of 1 year, 3 years and 5 years were 55.6%, 34.3% and 22.2%; respectively, OS was 93%, 61.1% and 49.7%. Univariate analysis: The location, depth, grade and treatment of tumor were related to PFS. Tumor depth, ulceration, grade and treatment were related to OS. Multivariate analysis: tumor depth and treatment were independent prognostic factors of PFS; tumor depth, ulceration and grade were independent prognostic factors of OS. Conclusions Angiosarcoma is based on surgical treatment, but blind and unplanned surgery should be avoided. Neoadjuvant therapy can benefit patients. Chemotherapy is dominated by taxanes and anthracyclines. For locally advanced patients, preoperative transformation therapy can be performed and radiotherapy is helpful to improve the local control rate. We should pay attention to the comprehensive treatment of surgery, chemotherapy and radiotherapy. -
Key words:
- angiosarcoma /
- soft tissue sarcoma (STS) /
- surgery /
- chemotherapy /
- radiotherapy
-
表 1 OS的影响因素
影响因素 例数(%) 中位总生存时间(月) 95%CI P 性别 0.553 男 28(65.1) 52 20.44~83.56 女 15(34.9) 24 0~93.57 年龄(岁) 0.874 <60 23(53.5) 52 0~107.16 ≥60 20(46.5) 71 27.38~114.62 部位 0.551 躯干 14(32.6) 77 0~197.98 四肢 13(30.2) 112 23.25~200.75 头皮 16(37.2) 35 18.31~51.69 肿瘤大小(cm) 0.193 ≤5 24(55.8) 71 − >5 19(44.2) 43 16.85~69.15 肿瘤深度 0.040 深 18(41.9) 24 10.04~37.96 浅 25(58.1) 77 19.91~143.09 肿瘤破溃 0.029 有 12(27.9) 35 7.94~62.06 无 31(72.1) 112 35.35~188.64 分级 0.020 G1+G2 15(34.9) − − G3 28(65.1) 31 15.60~46.39 治疗方式 0.022 非手术 10(23.3) 31 15.90~32.10 局部切除术 13(30.2) 24 50.17~103.83 扩大切除术 20(46.5) 77 13.14~90.86 转移 0.302 无 33(76.7) 77 22.87~131.13 有 10(23.3) 43 2.94~83.06 表 2 PFS的影响因素
影响因素 例数(%) 中位生存时间(月) 95%CI P 性别 0.816 男 28(65.1) 19 9.07~28.93 女 15(34.9) 12 2.91~21.09 年龄(岁) 0.525 <60 23(53.5) 21 7.16~34.85 ≥60 20(46.5) 13 0.93~25.07 部位 0.005 躯干 14(32.6) 6 0~13.33 四肢 13(30.2) 60 − 头皮 16(37.2) 15 4.17~25.83 肿瘤大小(cm) 0.813 ≤5 24(55.8) 13 0~26.64 >5 19(44.2) 16 0~32.06 肿瘤深度 0.006 深 18(41.9) 6 1.87~10.13 浅 25(58.1) 22 17.97~26.03 肿瘤破溃 0.723 有 12(27.9) 15 3.12~26.88 无 31(72.1) 16 3.39~28.61 分级 0.022 G1+G2 15(34.9) 23 0~47.22 G3 28(65.1%) 7 0~15.89 治疗方式 <0.001 非手术 10(23.3) 9 0~18.54 局部切除术 13(30.2) 3 0.99~5.01 扩大切除术 20(46.5) 39 0~84.29 表 3 Cox多因素分析
项目 HR 95%CI P 下限 上限 肿瘤深度(浅度vs. 深度) 0.321 0.104 0.987 0.047 治疗方式 非手术 vs. 局部切除术 3.975 1.368 11.549 0.011 无手术 vs. 扩大切除术 6.091 2.345 15.821 <0.001 表 4 Cox多因素分析(总生存)
项目 HR 95%CI P 下限 上限 分级(G1+G2 vs. G3) 0.222 0.062 0.797 0.021 肿瘤深度(浅 vs. 深) 0.222 0.061 0.806 0.022 肿瘤破溃(无 vs. 有) 0.094 0.025 0.356 <0.001 -
[1] Fayette J, Martin E, Piperno-Neumann S, et al. Angiosarcomas, a heterogeneous group of sarcomas with specific behavior depending on primary site: a retrospective study of 161 cases[J]. Ann Oncol, 2007, 18(12):2030-2036. doi: 10.1093/annonc/mdm381 [2] Florou V, Wilky BA. Current management of angiosarcoma: recent advances and lessons from the past[J]. Curr Treat Options Oncol, 2021, 22(7):61. doi: 10.1007/s11864-021-00858-9 [3] Penel N, Bui BN, Bay JO, et al. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX Study[J]. J Clin Oncol, 2008, 26(32):5269-5274. doi: 10.1200/JCO.2008.17.3146 [4] Ihara H, Kaji T, Katsui K, et al. Single institutional experience of radiation therapy for angiosarcoma of the scalp without cervical lymph node metastases: impact of concurrent chemoradiation with maintenance chemotherapy using taxanes on patient prognosis[J]. Mol Clin Oncol, 2019, 11(5):498-504. [5] von Mehren M, Kane JM, Bui MM, et al. NCCN guidelines insights: soft tissue sarcoma, version 1.2021[J]. J Natl Compr Canc Netw, 2020, 18(12):1604-1612. doi: 10.6004/jnccn.2020.0058 [6] Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1)[J]. Eur J Cancer, 2009, 45(2):228-247. doi: 10.1016/j.ejca.2008.10.026 [7] Ogawa K, Takahashi K, Asato Y, et al. Treatment and prognosis of angiosarcoma of the scalp and face: a retrospective analysis of 48 patients[J]. Br J Radiol, 2012, 85(1019):e1127-e1133. doi: 10.1259/bjr/31655219 [8] Depla AL, Scharloo-Karels CH, Jong MD, et al. Treatment and prognostic factors of radiation-associated angiosarcoma (RAAS) after primary breast cancer: a systematic review[J]. Eur J Cancer, 2014, 50(10):1779-1788. doi: 10.1016/j.ejca.2014.03.002 [9] Trofymenko O, Curiel-Lewandrowski C. Surgical treatment associated with improved survival in patients with cutaneous angiosarcoma[J]. J Eur Acad Dermatol Venereol, 2018, 32(1):e29-e31. doi: 10.1111/jdv.14479 [10] Singla S, Papavasiliou P, Powers B, et al. Challenges in the treatment of angiosarcoma: a single institution experience[J]. Am J Surg, 2014, 208(2):254-259. doi: 10.1016/j.amjsurg.2014.01.007 [11] Bernstein JM, Irish JC, Brown DH, et al. Survival outcomes for cutaneous angiosarcoma of the scalp versus face[J]. Head Neck, 2017, 39(6):1205-1211. doi: 10.1002/hed.24747 [12] Italiano A, Cioffi A, Penel N, et al. Comparison of doxorubicin and weekly paclitaxel efficacy in metastatic angiosarcomas[J]. Cancer, 2012, 118(13):3330-3336. doi: 10.1002/cncr.26599 [13] Ray-Coquard IL, Domont J, Tresch-Bruneel E, et al. Paclitaxel given once per week with or without bevacizumab in patients with advanced angiosarcoma: a randomized phase II trial[J]. J Clin Oncol, 2015, 33(25):2797-2802. doi: 10.1200/JCO.2015.60.8505 [14] Penel N, Italiano A, Ray-Coquard I, et al. Metastatic angiosarcomas: doxorubicin-based regimens, weekly paclitaxel and metastasectomy significantly improve the outcome[J]. Ann Oncol, 2012, 23(2):517-523. doi: 10.1093/annonc/mdr138 [15] Heinhuis KM, IJzerman NS, van der Graaf WTA, et al. Neoadjuvant systemic treatment of primary angiosarcoma[J]. Cancers, 2020, 12(8):2251. doi: 10.3390/cancers12082251 [16] Maki RG, D'Adamo DR, Keohan ML, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas[J]. J Clin Oncol, 2009, 27(19):3133-3140. doi: 10.1200/JCO.2008.20.4495 [17] Kollár A, Jones RL, Stacchiotti S, et al. Pazopanib in advanced vascular sarcomas: an EORTC Soft Tissue and Bone Sarcoma Group (STBSG) retrospective analysis[J]. Acta Oncol, 2017, 56(1):88-92. doi: 10.1080/0284186X.2016.1234068 [18] Agulnik M, Yarber JL, Okuno SH, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas[J]. Ann Oncol, 2013, 24(1):257-263. doi: 10.1093/annonc/mds237 -



 下载:
下载: