Efficacy and safety of anlotinib combined with adriamycin ifosfamide in the treatment of lung metastasis of soft tissue sarcoma
-
摘要:
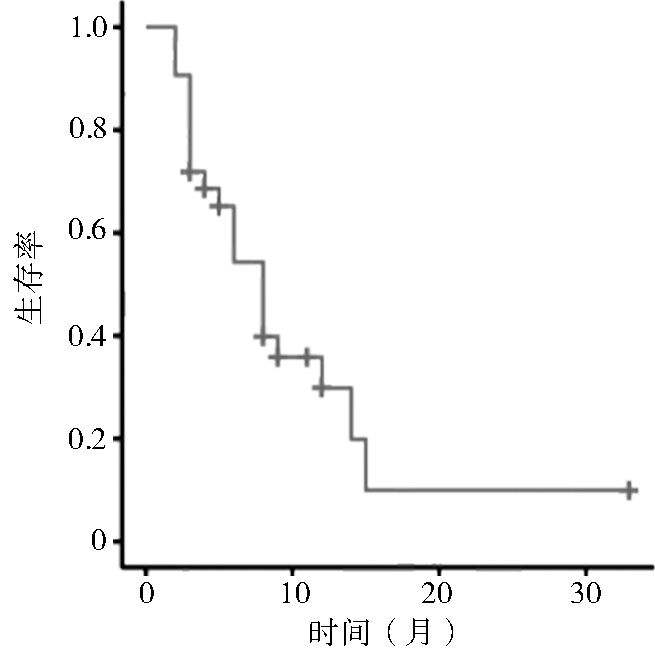
目的 回顾性研究阿霉素和异环磷酰胺(adriamycin ifosfamide,AI)方案化疗联合安罗替尼维持治疗对软组织肉瘤肺转移患者的疗效与安全性。 方法 收集天津医科大学肿瘤医院2018年6月至2022年4月软组织肉瘤肺转移接受AI方案化疗联合安罗替尼维持性治疗的32例患者。按照实体瘤疗效评价标准 RECIST 1.1进行治疗效果评价,计算客观缓解率(objective response rate,ORR)及疾病控制率(disease control rate,DCR),统计分析中位无进展生存期(median progression-free survival,mPFS)、中位总生存期(median overall survival,mOS)及相关不良事件。 结果 32例软组织肉瘤肺转移患者中完全缓解(complete response,CR)1例(3.1%),部分缓解(partial response,PR)10例(31.2%),病情稳定(stable disease,SD)9例(28.1%),疾病进展(progress disease,PD)12例(37.5%),ORR为34.3%,DCR为62.5%,mPFS为6.0个月,mOS为15.0个月。绝大多数患者治疗相关不良事件均为1~2级,3~4级不良事件包括胸腔积液(n=2,6.25%)、贫血(n=5,15.6%)、白细胞减少(n=4,12.5%)等,5级不良事件为气胸1人(3.1%)。 结论 AI化疗方案联合安罗替尼维持治疗对软组织肉瘤肺转移患者有一定效果,不良事件大部分患者可耐受,可作为软组织肉瘤肺转移的一种有效治疗手段。 Abstract:Objective To retrospectively study the efficacy and safety of adriamycin ifosfamide (AI) combined with anlotinib maintenance therapy in patients with lung metastasis of soft tissue sarcoma. Methods Patients with lung metastasis of soft tissue sarcoma treated in the tumor hospital of Tianjin Medical University from June 2018 to April 2022 were collected. They received AI and anlotinib maintenance treatment. The treatment effect was evaluated according to the solid tumor efficacy evaluation standard (RECIST) 1.1, the objective response rate (ORR) and disease control rate (DCR) were calculated, and the median progression-free survival (mPFS), median overall survival (mOS) and related adverse events were statistically analyzed. Results A total of 32 patients with lung metastasis of soft tissue sarcoma were included. One patient achieved complete response (CR) (3.1%), and 10 had partial response (PR) (31.2%), 9 had stable disease (SD) (28.1%), 12 had progress disease (PD) (37.5%); ORR was 34.3%, DCR was 62.5%. The mPFS was 6.0 months and mOS was 15.0 months. The treatment-related adverse events of most patients were grade 1-2. Grade 3-4 adverse events included pleural effusion (n=2, 6.25%), anemia (n=5, 15.6%), leucopenia (n=4, 12.5%). Grade 5 adverse events were pneumothorax (n=1, 3.1%). Conclusions AI combined with anlotinib maintenance therapy has a certain effect on patients with lung metastasis of soft tissue sarcoma. Most patients can tolerate the adverse events, which can be used as an effective treatment for lung metastasis of soft tissue sarcoma. -
Key words:
- soft tissue sarcoma /
- lung metastasis /
- adriamycin ifosfamide (AI) /
- anlotinib
-
表 1 患者一般资料
临床特点 例数(%) 性别 男 23(71.90) 女 9(28.10) ECOG评分(分) 0 18(56.30) 1 14(43.70) 病理亚型 多形性未分化肉瘤 9(28.10) 滑膜肉瘤 7(21.90) 上皮样肉瘤 5(15.60) 横纹肌肉瘤 3(9.40) 脂肪肉瘤 2(6.25) 上皮样血管肉瘤 2(6.25) 平滑肌肉瘤 2(6.25) 纤维肉瘤 1(3.10) 恶性外周神经鞘膜瘤 1(3.10) 原发灶部位 上肢 8(25.00) 下肢 15(46.90) 躯干 6(18.70) 腹膜后 3(9.40) 原发灶手术情况 是 30(93.75) 否 2(6.25) 是否放疗 是 8(25.00) 否 24(75.00) 化疗周期(个) 1 1(3.10) 2 3(9.30) 3 2(6.20) 4 9(28.10) 5 7(21.80) 6 10(31.30) 肺外转移灶 骨 6(18.70) 肝脏 1(3.10) 淋巴结 3(9.40) 肾上腺 1(3.10) 脑 1(3.10) 表 2 治疗效果评估
治疗效果 例数(%) CR 1(3.1) PR 10(31.3) SD 9(28.1) PD 12(37.5) 表 3 32例软组织肉瘤肺转移患者治疗效果及生存期
编号 年龄(岁) 性别 病理类型 治疗效果 PFS(月) OS(月) 生存状态 肿瘤最大径变化(%) 1 66 女 多形性未分化肉瘤 CR 33 33 健在 −100.0 2 71 女 多形性未分化肉瘤 PR 13 27 死亡 −52.7 3 54 男 上皮样肉瘤 PR 8 15 健在 −36.8 4 57 女 多形性未分化肉瘤 PR 9 17 健在 −40.9 5 26 男 横纹肌肉瘤 PR 11 40 健在 −31.2 6 34 女 滑膜肉瘤 PR 3 16 死亡 −33.8 7 63 男 多形性未分化肉瘤 PR 12 24 健在 −30.0 8 64 男 多形性未分化肉瘤 PR 4 24 健在 −74.3 9 29 男 恶性外周神经鞘膜瘤 PR 21 36 死亡 −58.3 10 55 男 滑膜肉瘤 PR 14 21 死亡 −42.7 11 57 男 多形性未分化肉瘤 PR 11 32 健在 −36.3 12 29 男 血管肉瘤 SD 12 38 死亡 2.8 13 46 女 滑膜肉瘤 SD 8 22 死亡 −28.6 14 70 男 横纹肌肉瘤 SD 6 13 死亡 8.4 15 23 男 上皮样肉瘤 SD 8 14 死亡 3.4 16 64 男 脂肪肉瘤 SD 12 15 死亡 −13.8 17 59 女 平滑肌肉瘤 SD 9 32 健在 −7.1 18 62 男 脂肪肉瘤 SD 3 11 死亡 16.8 19 51 女 平滑肌肉瘤 SD 8 29 死亡 18.2 20 61 男 多形性未分化肉瘤 SD 3 9 健在 5.7 21 58 男 滑膜肉瘤 PD 3 12 死亡 68.1 22 65 男 脂肪肉瘤 PD 2 4 死亡 52.3 23 75 男 横纹肌肉瘤 PD 2 4 死亡 73.6 24 61 女 多形性未分化肉瘤 PD 3 7 死亡 57.1 25 47 女 纤维肉瘤 PD 6 11 死亡 128.6 26 38 女 上皮样肉瘤 PD 8 11 死亡 52.8 27 60 男 多形性未分化肉瘤 PD 3 20 死亡 157.0 28 52 男 滑膜肉瘤 PD 3 13 健在 38.5 29 65 男 血管肉瘤 PD 6 12 死亡 28.5 30 54 男 滑膜肉瘤 PD 4 12 死亡 38.4 31 43 男 上皮样肉瘤 PD 3 6 死亡 630.0 32 71 男 上皮样肉瘤 PD 2 6 死亡 135.2 表 4 不同类型软组织肉瘤肺转移治疗效果
病理类型 总数 CR PR SD PD 疾病
控制
率(%)平均
PFS
(月)平均
OS(月)多形性未分化肉瘤 9 1 5 1 2 77.7 10.1 21.4 滑膜肉瘤 7 0 2 1 4 42.9 5.3 14.3 上皮样肉瘤 5 0 1 1 3 40.0 5.8 14.4 横纹肌肉瘤 3 0 1 1 1 66.7 6.3 19.0 脂肪肉瘤 2 0 0 1 1 50.0 5.0 7.5 血管肉瘤 2 0 0 1 1 50.0 9.0 25.0 平滑肌肉瘤 2 0 0 2 0 100 8.5 30.5 纤维肉瘤 1 0 0 0 1 0 6.0 11.0 恶性外周神经鞘膜瘤 1 0 1 0 0 100 21.0 36.0 表 5 治疗相关不良事件统计
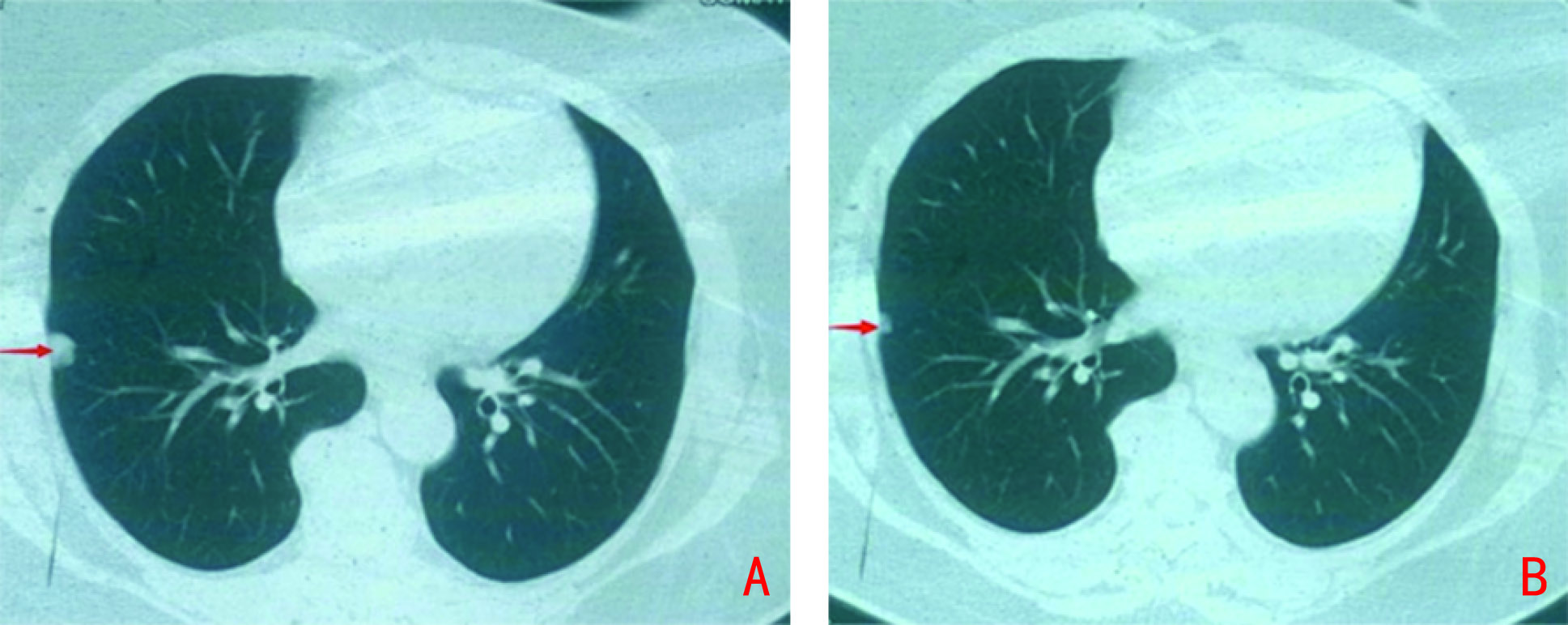
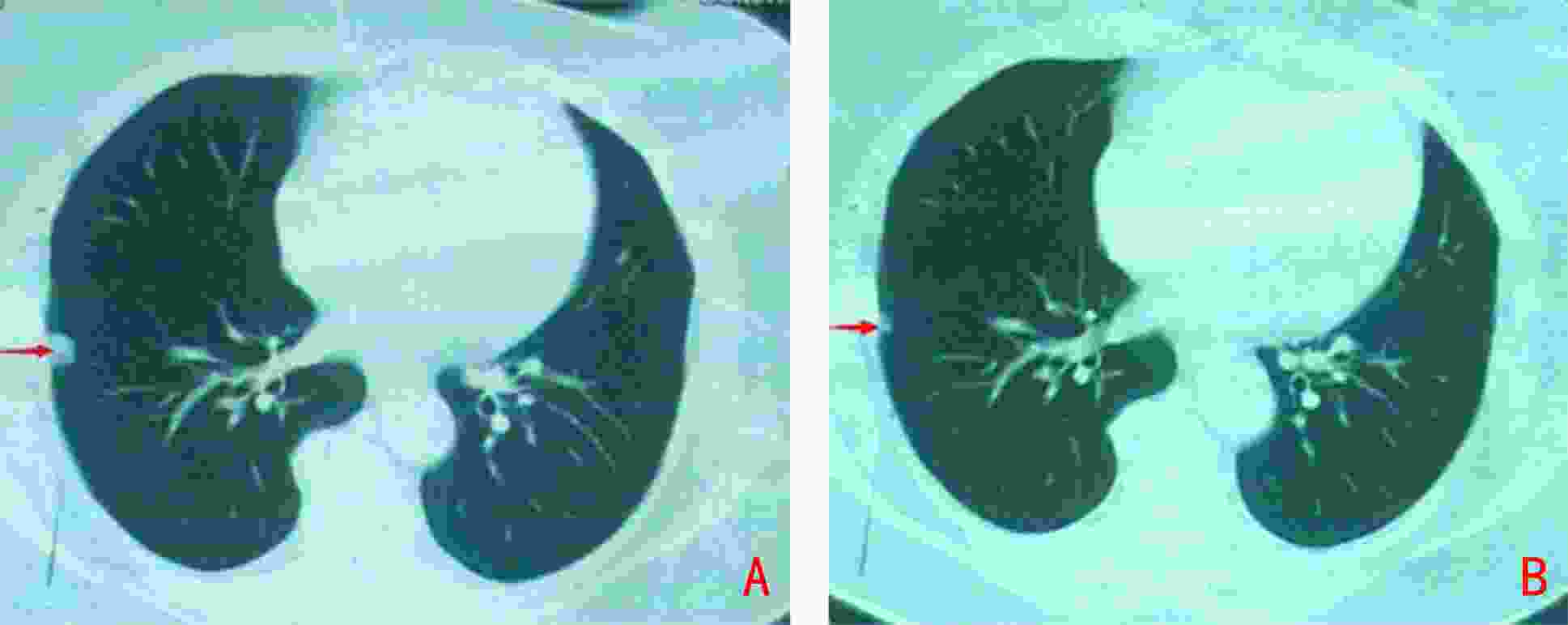
例(%) 项目 不良事件 总计 1~2级 3~4级 5级 过敏 1(3.10) 0(0) 0(0) 1(3.10) 手足综合征 4(12.50) 0(0) 0(0) 4(12.50) 口腔黏膜反应 4(12.50) 0(0) 0(0) 4(12.50) 乏力 4(12.50) 0(0) 0(0) 4(12.50) 失眠 2(6.25) 0(0) 0(0) 2(6.25) 高血压 10(31.30) 2(6.20) 0(0) 12(37.50) 骨骼肌肉疼痛 3(9.40) 1(3.10) 0(0) 4(12.50) 咳嗽 4(12.50) 0(0) 0(0) 4(12.50) 声嘶 2(6.25) 0(0) 0(0) 2(6.25) 咯血 3(9.40) 0(0) 0(0) 3(9.40) 胸闷 3(9.40) 0(0) 0(0) 3(9.40) 气胸 1(3.10) 1(3.10) 1(3.10) 3(9.40) 恶心呕吐 20(62.50) 6(18.80) 0(0) 26(81.30) 食欲下降 6(18.75) 0(0) 0(0) 6(18.75) 腹泻 3(9.40) 1(3.10) 0(0) 4(12.50) 便秘 3(9.40) 0(0) 0(0) 3(9.40) 皮下出血 1(3.10) 0(0) 0(0) 1(3.10) 贫血 14(43.80) 5(15.60) 0(0) 19(59.40) 白细胞减少 6(18.80) 4(12.50) 0(0) 10(31.30) 血小板降低 3(9.40) 1(3.10) 0(0) 4(12.50) 血浆白蛋白下降 14(43.80) 0(0) 0(0) 14(43.80) 血甘油三酯升高 4(12.50) 3(9.30) 0(0) 7(21.80) 甲功减退 1(3.10) 0(0) 0(0) 1(3.10) 蛋白尿 12(37.50) 2(6.25) 0(0) 14(43.75) ALT升高 6(18.80) 1(3.10) 0(0) 7(21.90) AST升高 8(25.00) 0(0) 0(0) 8(25.00) -
[1] Meyer M, Seetharam M. First-line therapy for metastatic soft tissue sarcoma[J]. Curr Treat Options Oncol, 2019, 20(1):6. doi: 10.1007/s11864-019-0606-9 [2] Kang S, Kim HS, Kim S, et al. Post-metastasis survival in extremity soft tissue sarcoma: a recursive partitioning analysis of prognostic factors[J]. Eur J Cancer, 2014, 50(9):1649-1656. doi: 10.1016/j.ejca.2014.03.003 [3] Lindner LH, Litière S, Sleijfer S, et al. Prognostic factors for soft tissue sarcoma patients with lung metastases only who are receiving first-line chemotherapy: an exploratory, retrospective analysis of the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STBSG)[J]. Int J Cancer, 2018, 142(12):2610-2620. doi: 10.1002/ijc.31286 [4] Judson I, Verweij J, Gelderblom H, et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial[J]. Lancet Oncol, 2014, 15(4):415-423. doi: 10.1016/S1470-2045(14)70063-4 [5] Shen GS, Zheng FC, Ren DF, et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development[J]. J Hematol Oncol, 2018, 11(1):120. doi: 10.1186/s13045-018-0664-7 [6] Yuan J, Li XY, Yu SJ. Molecular targeted therapy for advanced or metastatic soft tissue sarcoma[J]. Cancer Control, 2021, 28:1-20. [7] Chi Y, Fang ZW, Hong XN, et al. Safety and efficacy of anlotinib, a multikinase angiogenesis inhibitor, in patients with refractory metastatic soft-tissue sarcoma[J]. Clin Cancer Res, 2018, 24(21):5233-5238. doi: 10.1158/1078-0432.CCR-17-3766 [8] Vo KT, Matthay KK, DuBois SG. Targeted antiangiogenic agents in combination with cytotoxic chemotherapy in preclinical and clinical studies in sarcoma[J]. Clin Sarcoma Res, 2016, 6(1):1-11. doi: 10.1186/s13569-016-0041-7 [9] Zhou CC, Wu YL, Chen GY, et al. BEYOND: a randomized, double-blind, placebo-controlled, multicenter, phase Ⅲ study of first-line carboplatin/paclitaxel plus bevacizumab or placebo in Chinese patients with advanced or recurrent nonsquamous non-small-cell lung cancer[J]. J Clin Oncol, 2015, 33(19):2197-2204. doi: 10.1200/JCO.2014.59.4424 [10] Verschraegen CF, Arias-Pulido H, Lee SJ, et al. Phase ⅠB study of the combination of docetaxel, gemcitabine, and bevacizumab in patients with advanced or recurrent soft tissue sarcoma: the Axtell regimen[J]. Ann Oncol, 2012, 23(3):785-790. doi: 10.1093/annonc/mdr299 [11] del Muro XG, Maurel J, Trufero JM, et al. Phase Ⅱ trial of ifosfamide in combination with the VEGFR inhibitor sorafenib in advanced soft tissue sarcoma: a Spanish group for research on sarcomas (GEIS) study[J]. Invest New Drugs, 2018, 36(3):468-475. doi: 10.1007/s10637-018-0583-z [12] Liu J, Deng YT, Jiang Y. Switch maintenance therapy with anlotinib after chemotherapy in unresectable or metastatic soft tissue sarcoma: a single-center retrospective study[J]. Invest New Drugs, 2021, 39(2):330-336. doi: 10.1007/s10637-020-01015-z [13] Wang HY, Chu JF, Zhang P, et al. Safety and efficacy of chemotherapy combined with anlotinib plus anlotinib maintenance in Chinese patients with advanced/metastatic soft tissue sarcoma[J]. Onco Targets Ther, 2020, 13:1561-1568. doi: 10.2147/OTT.S235349 [14] Li SL. Anlotinib: a novel targeted drug for bone and soft tissue sarcoma[J]. Front Oncol, 2021, 11:664853. [15] Bense L, Eklund G, Wiman LG. Smoking and the increased risk of contracting spontaneous pneumothorax[J]. Chest, 1987, 92(6):1009-1012. doi: 10.1378/chest.92.6.1009 -



 下载:
下载: