Clinical outcomes of acute myeloid leukemia with genetically poor prognosis: a real-world study
-
摘要:
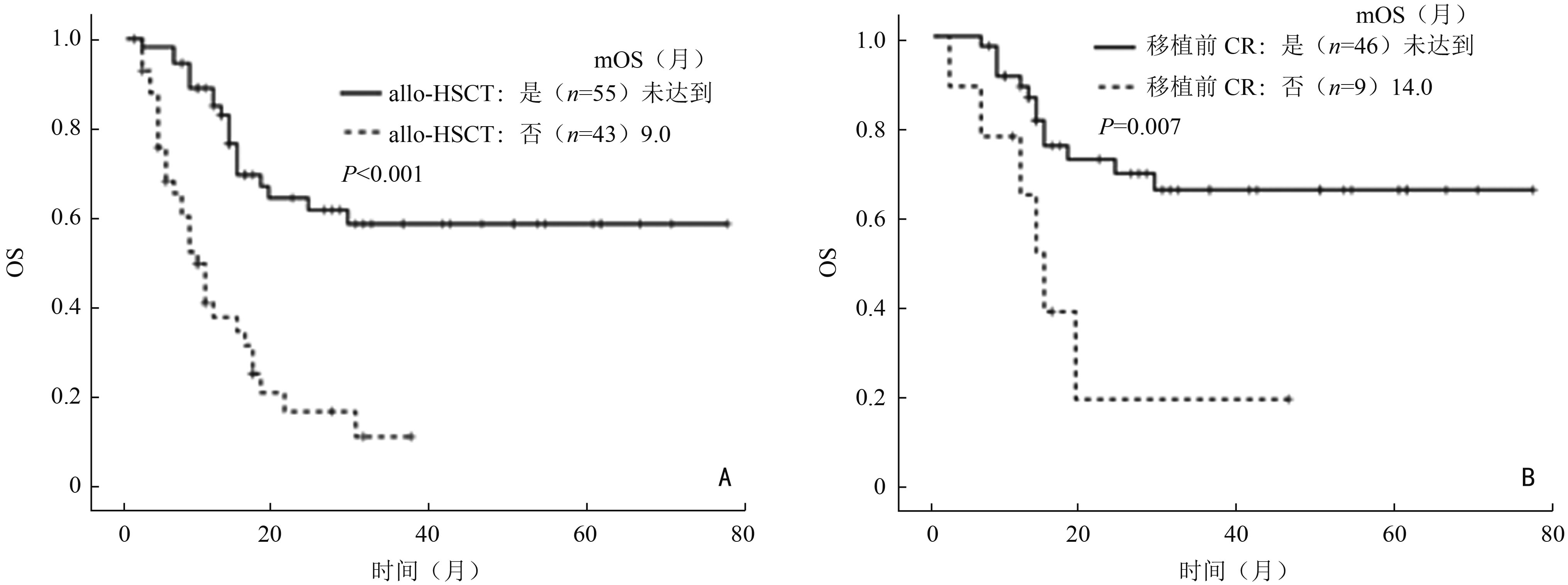
目的 观察标准化疗诱导的预后不良组急性髓系白血病(acute myeloid leukemia, AML)的临床特征。 方法 回顾性分析南方医科大学珠江医院2016年1月至2020年12月收治的98例首个疗程使用标准方案化疗的预后不良组AML患者的临床和实验室资料。 结果 预后不良组AML患者中位年龄为37(18~67)岁,其中男性占61.2%。复杂核型(30.0%)、+8(20.0%)、单体核型(18.9%)是最常见的细胞遗传学异常,FLT3-ITD(34.1%)、ASXL1(20.5%)、RUNX1(15.9%)、DNMT3A(15.9%)、TET2(15.9%)是最常见的基因突变,其首疗程完全缓解率为37.8%。中位总生存期(median overall survival,mOS)和中位无事件生存期(median event-free survival,mEFS)分别是17个月和6个月,中位无复发生存期(median relapse-free survival, mRFS)为12个月。移植方面,有55例患者进行了异基因造血干细胞移植,中位随访16个月,mOS尚未达到,显著高于未移植组的9个月(P<0.001)。移植后1年及2年累积复发率分别是22.7%及31.8%,1年的非复发死亡率为15.8%。此外,对于34例首个疗程诱导后评估为未缓解的患者,不同方案的治疗效果比较显示:CAG±去甲基化药物方案相较于标准“3+7”方案具有更高的再诱导完全缓解率(77.8% vs. 23.1%;P=0.027)。 结论 预后不良组AML患者缓解率低,复发率高,生存期短。对于首疗程诱导化疗效果较差的预后不良组AML,CAG联合去甲基化药物是一种可考虑的再诱导方案。 Abstract:Objective To evaluate the clinical outcomes of acute myeloid leukemia (AML) with poor prognosis induced by standard chemotherapy. Methods The clinical and laboratory data of 98 AML patients with poor prognosis treated with standard chemotherapy during their first induction therapy who were admitted at Zhujiang Hospital of Southern Medical University from January 2016 to December 2020 were retrospectively analyzed. Results In the AML patients with poor prognosis, the mean age was 37 (18–67) years, and 61.2% were men. Complex karyotype (30.0%), +8 (20.0%) and monosomal karyotype (18.9%) were the most common cytogenetic abnormalities. Besides, FMS-like tyrosine kinase 3-internal tandem duplications (FLT3-ITD) (34.1%), additional sex combs like-1 (ASXL1) (20.5%), runt-related transcription factor 1 (RUNX1) (15.9%), DNA methyltransferase 3 alpha (DNMT3A) (15.9%), and TET methylcytosine dioxygenase 2 (TET2) (15.9%) were the most common gene mutations. The complete remission rate following the first course of treatment was 37.8%. The median overall survival (mOS) and median event-free survival (mEFS) times were 17.0 and 6.0 months, respectively. The median relapse-free survival (mRFS) was 12.0 months. In terms of transplantation, 55 patients had undergone allogeneic hematopoietic stem cell transplantation with a median follow-up of 16.0 months, while the mOS was not yet reached, which was significantly higher than 9.0 months in patients who did not undergo transplantation (P< 0.001). The analyses of relapse after transplantation showed that the cumulative relapse rates at 1 and 2 years after transplantation were 22.7% and 31.8%, respectively. The non-relapse mortality rate at 1 year after transplantation was 15.8%. In 34 AML patients with no remission after the first course of induction therapy, the comparison of the therapeutic effects of different regimens showed that treatment with CAG±hypomethylating agents showed better reinduced complete remission compared with the standard “3+7” regimens (77.8% vs. 23.1%; P=0.027). Conclusions AML patients assigned to the poor prognosis group have low remission rate, high relapse rate, and short survival time. CAG combined with hypomethylating agents may be considered as a reinduction option for AML patients with poor prognosis who respond poorly to the first course of induction chemotherapy. -
表 1 预后不良AML患者基线特征
基线特征1 所有患者 行allo-HSCT 未行allo-HSCT P (n=98) (n=55) (n=43) 男性(n,%) 60(61.2) 36(65.5) 24(55.8) 0.331 中位年龄(岁) 37(18~67) 34(18~58) 48(18~67) <0.001 继发性AML(n,%) 7(7.1) 1(1.8) 6(14.0) 0.041 实验室检查 白细胞计数(×109/L) 25.4(1.0~323.2) 23.6(1.0~323.2) 46.7(1.1~243.7) 0.656 血红蛋白含量(g/L) 75.5(14.0~137.0) 81.0(14.0~137.0) 67.5(31.0~111.0) 0.005 血小板计数(×109/L) 44.0(3.0~540.0) 48.0(3.0~234.0) 40.0(7.0~540.0) 0.635 原始细胞比例(%) 71.5(15.5~98.0) 79.0(24.0~98.0) 53.3(15.5~95.5) 0.004 染色体异常(n,%) 复杂核型 27(30.0) 13(26.5) 14(34.1) 0.432 +8 18(20.0) 11(22.4) 7(17.1) 0.525 单体核型 17(18.9) 7(14.3) 10(24.4) 0.223 t(v;11q23.3) 8(8.9) 6(12.2) 2(4.9) 0.283 −7 7(7.8) 2(4.1) 5(12.2) 0.239 −17/abn17 7(7.8) 3(6.1) 4(9.8) 0.698 t(3;3)(q21.3;q26.2) 6(6.7) 1(2.0) 5(12.2) 0.089 −5/−5q 4(4.4) 1(2.0) 3(7.3) 0.327 t(9;22) 2(2.2) 2(4.1) 0(0) 1.000 t(6;9)(p23;q34.1) 1(1.1) 1(2.0) 0(0) 1.000 分子学异常(n,%) FLT3-ITD 30(34.1) 20(40.0) 10(26.3) 0.180 ASXL1 18(20.5) 10(20.0) 8(21.1) 0.903 RUNX1 14(15.9) 6(12.0) 8(21.1) 0.250 DNMT3A 14(15.9) 7(14.0) 7(18.4) 0.574 TET2 14(15.9) 10(20.0) 4(10.5) 0.229 TP53 9(10.2) 3(6.0) 6(15.8) 0.166 IDH2 6(6.8) 4(8.0) 2(5.3) 0.695 NRAS 5(5.7) 4(8.0) 1(2.6) 0.384 KRAS 5(5.7) 2(4.0) 3(7.9) 0.648 PHF6 4(4.5) 3(6.0) 1(2.6) 0.631 IDH1 3(3.4) 2(4.0) 1(2.6) 1.000 KIT 3(3.4) 1(2.0) 2(5.3) 0.576 a:染色体异常和分子学异常临床数据存在少数缺失。对于染色体异常的计算,所有患者组、行allo-HSCT组和未行allo-HSCT组分母分别为90、49、41;对于分子学异常的计算,所有患者组、行allo-HSCT组和未行allo-HSCT组分母分别为88、50、38;P值:行allo-HSCT组和未行allo-HSCT组进行比较 表 2 预后不良AML患者OS的单因素和多因素分析
(n=98) 变量 单因素分析 多因素分析 HR(95%CI) P HR(95%CI) P 性别 0.794(0.452,1.393) 0.421 − − 年龄 1.032(1.010,1.054) 0.003 0.995(0.962,1.029) 0.775 继发性AML 5.543(2.219,13.846) <0.001 5.182(0.700,38.360) 0.107 实验室检查 白细胞计数 1.000(0.997,1.004) 0.809 − − 血小板计数 1.000(0.996,1.005) 0.910 − − 血红蛋白含量 0.991(0.978,1.003) 0.148 − − 原始细胞比例 0.989(0.976,1.002) 0.089 1.0(0.977,1.024) 0.967 染色体异常 复杂核型 1.983(1.101,3.571) 0.022 7.818(2.223,27.496) 0.001 +8 0.822(0.395,1.707) 0.598 0.181(0.052,0.625) 0.007 单体核型 1.814(0.938,3.506) 0.077 1.966(0.376,10.277) 0.423 t(v;11q23.3) 0.958(0.343,2.675) 0.935 3.753(0.855,16.485) 0.080 −7 2.513(1.057,5.972) 0.037 0.275(0.044,1.720) 0.168 −17/abn17 2.168(0.917,5.122) 0.078 0.282(0.041,1.948) 0.199 t(3;3)(q21.3;q26.2) 2.815(1.093,7.250) 0.032 1.389(0.182,10.600) 0.751 −5/−5q 3.704(1.318,10.404) 0.013 − − t(9;22) 0.048(0,131.251) 0.451 − − t(6;9)(p23;q34.1) 0.048(0,487.727) 0.519 − − 分子学异常 FLT3-ITD 0.608(0.307,1.204) 0.153 2.346(0.744,7.395) 0.146 ASXL1 1.327(0.670,2.626) 0.417 6.170(1.557,24.443) 0.010 RUNX1 0.765(0.323,1.812) 0.542 1.337(0.341,5.250) 0.677 DNMT3A 1.256(0.556,2.836) 0.583 3.320(0.973,11.330) 0.055 TET2 0.836(0.353,1.980) 0.684 0.414(0.085,2.026) 0.277 TP53 2.588(1.147,5.843) 0.022 1.348(0.331,5.491) 0.677 IDH2 0.848(0.262,2.746) 0.783 1.345(0.242,7.477) 0.735 NRAS 0.625(0.151,2.587) 0.517 0.425(0.053,3.414) 0.421 KRAS 0.499(0.069,3.635) 0.493 0.047(0.004,0.610) 0.019 PHF6 1.896(0.586,6.134) 0.285 − − IDH1 0.709(0.097,5.150) 0.734 − − KIT 4.590(1.382,15.242) 0.013 − − 异基因造血干细胞移植 0.243(0.136,0.436) <0.001 0.224(0.081,0.621) 0.004 表 3 首个疗程后评估为NR的AML患者再诱导CR率的对比(n=34)
组别 CR率(%) CAG±HMA vs. 标准方案 CAG±HMA 77.8(7/9) 标准方案 23.1(3/13) P 0.027 强化方案 vs. 标准方案 强化方案 50.0(6/12) 标准方案 23.1(3/13) P 0.226 -
[1] Xuan L, Liu QF. Maintenance therapy in acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation[J]. J Hematol Oncol, 2021, 14(1):4. doi: 10.1186/s13045-020-01017-7 [2] Estey EH. How to manage high-risk acute myeloid leukemia[J]. Leukemia, 2012, 26(5):861-869. doi: 10.1038/leu.2011.317 [3] NCCN guidelines insights:acute myeloid leukemia, Version 2. 2021[J]. J Natl Compr Canc Netw, 2021, 19(1):16-27. doi: 10.6004/jnccn.2021.0002 [4] Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel[J]. Blood, 2017, 129(4):424-447. doi: 10.1182/blood-2016-08-733196 [5] 中华医学会血液学分会白血病淋巴瘤学组,王建祥,魏辉.中国成人急性髓系白血病(非急性早幼粒细胞白血病)诊疗指南(2021年版)[J].中华血液学杂志,2021,42(8):617-623. doi: 10.3760/cma.j.issn.0253-2727.2021.08.001 [6] Palmieri R, Othus M, Halpern AB, et al. Accuracy of SIE/SIES/GITMO consensus criteria for unfitness to predict early mortality after intensive chemotherapy in adults with AML or other high-grade myeloid neoplasm[J]. J Clin Oncol, 2020, 38(35):4163-4174. [7] Cheson BD, BennettJM, Kopecky KJ, et al. Revised recommendations of the international working group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia[J]. J Clin Oncol, 2003, 21(24):4642-4649. doi: 10.1200/JCO.2003.04.036 [8] 中华医学会血液学分会白血病淋巴瘤学组.复发难治性急性髓系白血病中国诊疗指南(2017年版)The guidelines for diagnosis and treatment of acute myelogenous leukemia (relapse/refractory)rin China (2017)[J].中华血液学杂志,2017,38(3):183-184. doi: 10.3760/cma.j.issn.0253-2727.2017.03.002 [9] Ruggeri A, Labopin M, Sanz G, et al. Comparison of outcomes after unrelated cord blood and unmanipulated haploidentical stem cell transplantation in adults with acute leukemia[J]. Leukemia, 2015, 29(9):1891-1900. doi: 10.1038/leu.2015.98 [10] Xu LP, Chen H, Chen J, et al. The consensus on indications, conditioning regimen, and donor selection of allogeneic hematopoietic cell transplantation for hematological diseases in China-recommendations from the Chinese Society of Hematology[J]. J Hematol Oncol, 2018, 11(1):33. doi: 10.1186/s13045-018-0564-x [11] Bejanyan N, Weisdorf DJ, Logan BR, et al. Survival of patients with acute myeloid leukemia relapsing after allogeneic hematopoietic cell transplantation: a center for international blood and marrow transplant research study[J]. Biol Blood Marrow Transplant, 2015, 21(3):454-459. doi: 10.1016/j.bbmt.2014.11.007 [12] Gao L, Zhang YQ, Wang SB, et al. Effect of rhG-CSF combined with decitabine prophylaxis on relapse of patients with high-risk MRD-negative AML after HSCT: an open-label, multicenter, randomized controlled trial[J]. J Clin Oncol, 2020, 38(36):4249-4259. doi: 10.1200/JCO.19.03277 [13] CiureaSO, Labopin M, Socie G, et al. Relapse and survival after transplantation for complex karyotype acute myeloid leukemia: a report from the acute leukemia working party of the european society for blood and marrow transplantation and the university of texas MD anderson cancer center[J]. Cancer, 2018, 124(10):2134-2141. doi: 10.1002/cncr.31311 [14] Araki D, Wood BL, Othus M, et al. Allogeneic hematopoietic cell transplantation for acute myeloid leukemia: time to move toward a minimal residual disease-based definition of complete remission [J]? J Clin Oncol, 2016, 34(4): 329-336. [15] Oran B, Jorgensen JL, Marin D, et al. Pre-transplantation minimal residual disease with cytogenetic and molecular diagnostic features improves risk stratification in acute myeloid leukemia[J]. Haematologica, 2017, 102(1):110-117. doi: 10.3324/haematol.2016.144253 [16] Brissot E, Labopin M, Ehninger G, et al. Haploidentical versus unrelated allogeneic stem cell transplantation for relapsed/refractory acute myeloid leukemia: a report on 1578 patients from the acute leukemia working party of the EBMT[J]. Haematologica, 2019, 104(3):524-532. doi: 10.3324/haematol.2017.187450 [17] Guo HD, Chang YJ, Hong Y, et al. Dynamic immune profiling identifies the stronger graft-versus-leukemia (GVL) effects with haploidentical allografts compared to HLA-matched stem cell transplantation[J]. Cell Mol Immunol, 2021, 18(5):1172-1185. doi: 10.1038/s41423-020-00597-1 [18] BurnettAK, Russell NH, Hills RK, et al. A randomized comparison of daunorubicin 90 mg/m2 vs. 60 mg/m2 in AML induction: results from the UK NCRI AML17 trial in 1206 patients[J]. Blood, 2015, 125(25):3878-3885. doi: 10.1182/blood-2015-01-623447 [19] Teuffel O, Leibundgut K, Lehrnbecher T, et al. Anthracyclines during induction therapy in acute myeloid leukaemia: a systematic review and meta-analysis[J]. Br J Haematol, 2013, 161(2):192-203. doi: 10.1111/bjh.12233 [20] Fernandez HF, Sun Z, Yao X, et al. Anthracycline dose intensification in acute myeloid leukemia[J]. N Engl J Med, 2009, 361(13):1249-1259. doi: 10.1056/NEJMoa0904544 [21] Lim SJ, Lim MJ, Raptis A, et al. Inferior outcome after allogeneic transplant in first remission in high-risk AML patients who required more than two cycles of induction therapy[J]. Am J Hematol, 2015, 90(8):715-718. doi: 10.1002/ajh.24062 [22] Miyamura T, Moritake H, Nakayama H, et al. Clinical and biological features of paediatric acute myeloid leukaemia (AML) with primary induction failure in the Japanese paediatric leukaemia/lymphoma study group AML-05 study[J]. Br J Haematol, 2019, 185(2):284-288. doi: 10.1111/bjh.15799 [23] McHayleh W, Sehgal R, Redner RL, et al. Mitoxantrone and etoposide in patients with newly diagnosed acute myeloid leukemia with persistent leukemia after a course of therapy with cytarabine and idarubicin[J]. Leuk Lymphoma, 2009, 50(11):1848-1853. doi: 10.3109/10428190903216788 [24] Suzushima H, Wada N, Yamasaki H, et al. Low-dose cytarabine and aclarubicin in combination with granulocyte colony-stimulating factor for elderly patients with previously untreated acute myeloid leukemia[J]. LeukRes, 2010, 34(5):610-614. [25] Jin J, Chen J, Suo SS, et al. Low-dose cytarabine, aclarubicin and granulocyte colony-stimulating factor priming regimen versus idarubicin plus cytarabine regimen as induction therapy for older patients with acute myeloid leukemia[J]. Leuk Lymphoma, 2015, 56(6):1691-1697. doi: 10.3109/10428194.2014.963074 [26] 孙爱宁,孙妍珺,徐杨,等.地西他滨联合预激方案治疗53例复发难治正常核型急性髓系白血病的疗效分析[J].中华血液学杂志,2015(36):1025-1030. -



 下载:
下载: