Evaluation of efficacy of pyrotinib in patients with HER-2 negative advanced breast cancer and HER-2 mutation
-
摘要:
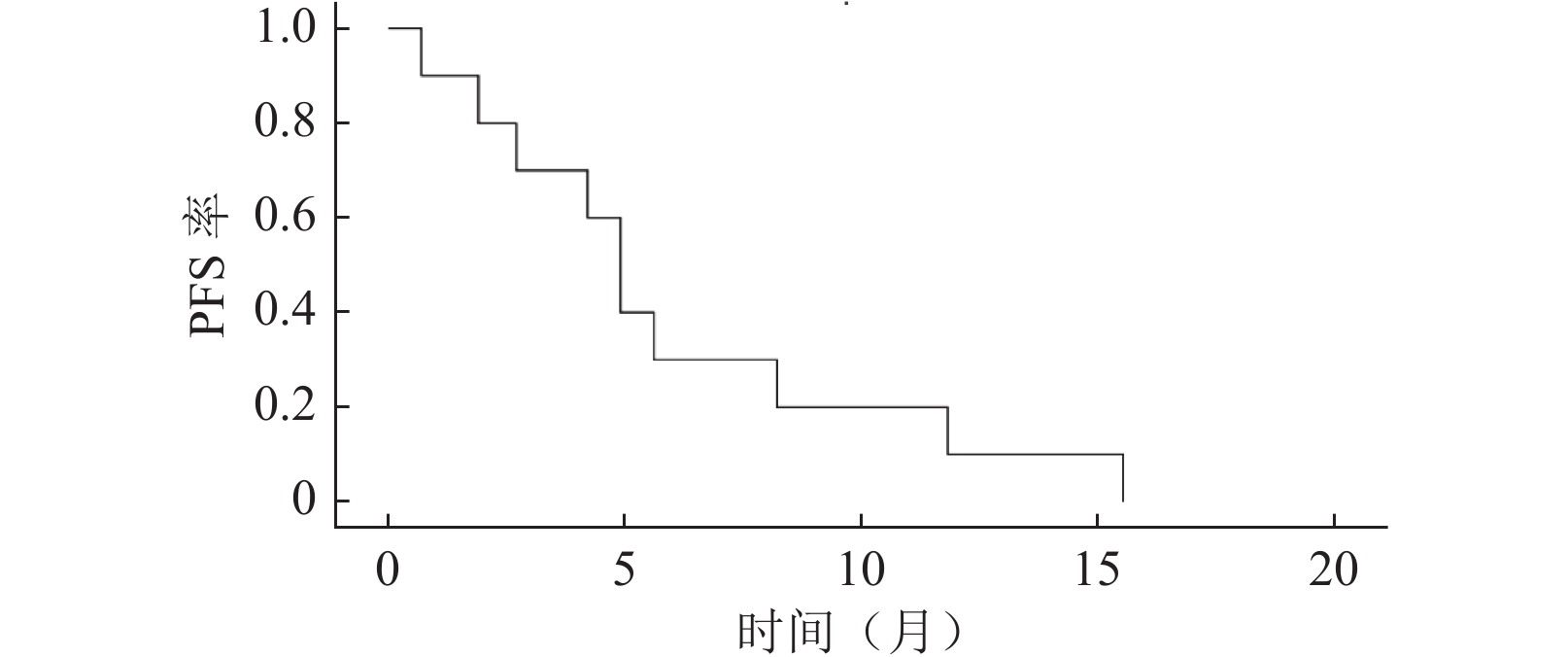
目的 评估吡咯替尼对携带HER-2突变的HER-2阴性晚期乳腺癌患者的治疗疗效和安全性。 方法 回顾性分析2018年1月至2019年6月于北京市朝阳区桓兴肿瘤医院收治的13例携带HER-2突变的HER-2阴性晚期乳腺癌患者的临床资料,均使用吡咯替尼单药行抗HER-2治疗,对患者的治疗疗效和不良反应进行综合评估。 结果 13例患者中3例因不良反应难以耐受出组,未行疗效评价,其余10例患者中获得完全缓解(complete response,CR)1例、部分缓解(partial response,PR)3例、疾病稳定(stable disease,SD)3例、疾病进展(progressive disease,PD)3例。客观缓解(CR+PR)率为40%(4/10),临床获益(CR+PR+SD≥6.0个月)率为60%(6/10),疾病控制(CR+PR+SD)率为70%(7/10)。无进展生存时间最长者达15.5个月,中位无进展生存期(progression-free survival,PFS)为4.9个月(95% CI:3.8~6.0)。吡咯替尼最常见的不良反应为腹泻、占84.6%(11/13),3级及以上的不良反应包括腹泻为15.4%(2/13)、恶心为7.7%(1/13)、呕吐为7.7%(1/13)。 结论 携带HER-2突变的HER-2阴性晚期乳腺癌患者,可能从吡咯替尼的抗HER-2治疗中获益。 Abstract:Objective To evaluate the efficacy and safety of pyrotinib in managing patients with HER-2 negative advanced breast cancer, with HER-2 mutation. Methods A retrospective analysis was performed using the clinical data of 13 patients with HER-2 negative advanced breast cancer, with HER-2 mutation, admitted to the Huan Xing Cancer Hospital, Chaoyang District, Beijing, from January 2018 to June 2019. All patients were managed with pyrotinib monotherapy against HER-2. The therapeutic efficacy and adverse events of the patients were comprehensively evaluated. Results Among the 13 patients, three were excluded from the study because of intolerant adverse events before the first tumor response evaluation. Among the ten remaining patients, one patient achieved a complete response (CR), three patients achieved partial response (PR), three patients achieved a best response of stable disease (SD), and three patients experienced progressive disease (PD). The objective response (CR+PR) rate was 40.0% (4/10), the clinical benefit (CR+PR+SD≥6.0 months) rate was 60% (6/10), and the disease control (CR+PR+SD) rate was 70% (7/10). The longest progression-free survival (PFS) time was 15.5 months, and the median PFS was 4.9 months (95% CI: 3.8-6.0). The main toxicity related to pyrotinib was diarrhea, accounting for 84.6% (11/13). Adverse events above grade 3 experienced by the patients included diarrhea (2/13, 15.4%), nausea (1/13, 7.7%), and vomiting (1/13, 7.7%). Conclusions Patients with HER-2 negative advanced breast cancer, with HER-2 mutation, may benefit from anti-HER-2 treatment with pyrotinib. -
Key words:
- HER-2 mutation /
- HER-2 negative /
- pyrotinib /
- advanced breast cancer
-
表 1 13例晚期乳腺癌患者的基因突变位点
患者序号 HER-2突变位点 合并PIK3CA
突变最佳疗效 1 p.L755_T759del 否 未评价 2 p.G776V 是 未评价 3 p.S310Y、p.L403V、p.E1021K、p.V659L 否 未评价 4 p.S310F 是 CR 5 p.L755S 是 PR 6 p.Y772_A775dup 否 PR 7 p.D769Y、p.T862A 否 PR 8 p.S310Y、p.L403V 是 SD 9 p.D769Y、p.V777L 否 SD 10 p.S310F、p.D769Y 是 SD 11 p.A1076T 否 PD 12 p.Y772_A775dup 否 PD 13 p.L767M 否 PD 表 2 13例晚期乳腺癌患者吡咯替尼治疗后出现的不良反应情况
不良反应 总计 不良反应≥3级 例数 百分比(%) 例数 百分比(%) 腹泻 11 84.6 2 15.4 呕吐 2 15.4 1 7.7 AST升高 2 15.4 0 0 食欲下降 2 15.4 0 0 口腔溃疡 1 7.7 0 0 恶心 1 7.7 1 7.7 肌酐升高 1 7.7 0 0 ALT升高 1 7.7 0 0 乏力 1 7.7 0 0 ALT:丙氨酸氨基转移酶;AST:天门冬氨酸氨基转移酶 -
[1] Khoury T, Mojica W, Hicks D, et al. ERBB2 juxtamembrane domain (trastuzumab binding site) gene mutation is a rare event in invasive breast cancers overexpressing the ERBB2 gene[J]. Mod Pathol, 2011, 24(8):1055-1059. doi: 10.1038/modpathol.2011.64 [2] Bose R, Kavuri SM, Searleman AC, et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer[J]. Cancer Discov, 2013, 3(2):224-237. doi: 10.1158/2159-8290.CD-12-0349 [3] Yi ZB, Rong GH, Guan YF, et al. Molecular landscape and efficacy of HER2-targeted therapy in patients with HER2-mutated metastatic breast cancer[J]. NPJ Breast Cancer, 2020, 6:59. doi: 10.1038/s41523-020-00201-9 [4] Zehir A, Benayed R, Shah RH, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10, 000 patients[J]. Nat Med, 2017, 23(8):1004. [5] 赵玮,边莉,江泽飞.HER-2基因突变在乳腺癌中的研究现状[J].中华乳腺病杂志(电子版),2016,10(6):362-365. [6] Ma CX, Luo JQ, Freedman RA, et al. The phase Ⅱ MutHER study of neratinib alone and in combination with fulvestrant in HER2-mutated, non-amplified metastatic breast cancer[J]. Clin Cancer Res, 2022, 28(7):1258-1267. doi: 10.1158/1078-0432.CCR-21-3418 [7] Zhang XY, Zhao WW, Wei W, et al. Parallel analyses of somatic mutations in plasma circulating tumor DNA (ctDNA) and matched tumor tissues in early-stage breast cancer[J]. Clin Cancer Res, 2019, 25(21):6546-6553. doi: 10.1158/1078-0432.CCR-18-4055 [8] Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors[J]. Nat Rev Cancer, 2005, 5(5):341-354. doi: 10.1038/nrc1609 [9] Prempree T, Wongpaksa C. Mutations of HER2 gene in HER2-positive metastatic breast cancer[J]. J Clin Oncol, 2006, 24(18_Suppl):13118. doi: 10.1200/jco.2006.24.18_suppl.13118 [10] Park YH, Shin HT, Jung HH, et al. Role of HER2 mutations in refractory metastatic breast cancers: targeted sequencing results in patients with refractory breast cancer[J]. Oncotarget, 2015, 6(31):32027-32038. doi: 10.18632/oncotarget.5184 [11] Hindson BJ, Ness KD, Masquelier DA, et al. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number[J]. Anal Chem, 2011, 83(22):8604-8610. doi: 10.1021/ac202028g [12] Ma F, Ouyang QC, Li W, et al. Pyrotinib or lapatinib combined with capecitabine in HER2-positive metastatic breast cancer with prior taxanes, anthracyclines, and/or trastuzumab: a randomized, phase II study[J]. J Clin Oncol, 2019, 37(29):2610-2619. doi: 10.1200/JCO.19.00108 [13] 宋国红,李惠平,邸立军,等.真实世界吡咯替尼治疗HER2阳性转移性乳腺癌的疗效及安全性[J].北京大学学报(医学版),2020,52(2):254-260. doi: 10.19723/j.issn.1671-167X.2020.02.010 [14] Ma F, Li Q, Chen SS, et al. Phase I study and biomarker analysis of pyrotinib, a novel irreversible pan-ErbB receptor tyrosine kinase inhibitor, in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer[J]. J Clin Oncol, 2017, 35(27):3105-3112. doi: 10.1200/JCO.2016.69.6179 [15] Li Q, Guan XW, Chen SS, et al. Safety, efficacy, and biomarker analysis of pyrotinib in combination with capecitabine in HER2-positive metastatic breast cancer patients: a phase I clinical trial[J]. Clin Cancer Res, 2019, 25(17):5212-5220. doi: 10.1158/1078-0432.CCR-18-4173 -



 下载:
下载:


