磷酸芦可替尼 依托泊苷联合DDGP方案在NK/T细胞淋巴瘤相关噬血细胞综合征初始诱导治疗的疗效及安全性研究
doi: 10.12354/j.issn.1000-8179.2023.20221557
Efficacy and safety of ruxolitinib phosphate and etoposide combined with DDGP as an initial induction regimen for patients with NK/T-cell lymphoma-associated hemophagocytic syndrome
-
摘要:
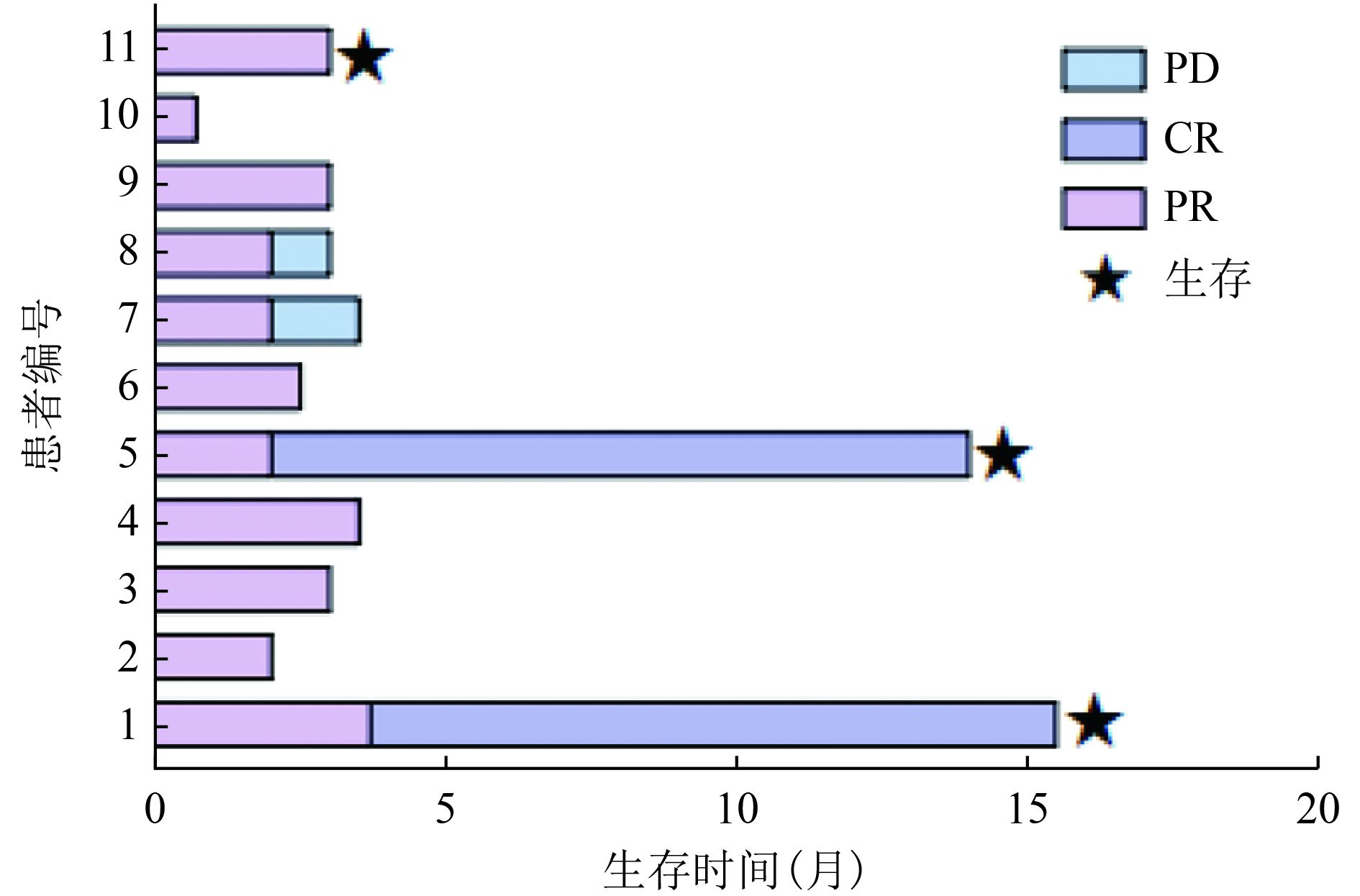
目的 探讨以磷酸芦可替尼、依托泊苷联合DDGP(RuE-DDGP)方案初始诱导治疗NK/T细胞淋巴瘤相关噬血细胞综合征(NK/T-cell lymphoma-associated hemophagocytic syndrome,NK/T-LAHS)的临床疗效及安全性。 方法 分析郑州大学第一附属医院2021年1月至2022年6月收治的11例采用RuE-DDGP方案治疗的NK/T-LAHS患者的临床资料,对其疗效、不良反应及预后进行回顾性分析。 结果 11例患者中9例为男性,2例为女性,中位年龄为30(20~75)岁。治疗2周后11例均获部分缓解(partial remission,PR),治疗4周后10例评效为PR,1例因死亡未评效。治疗2周后总体缓解率(overall remission rate,ORR)为100%,治疗4周后ORR为90.9%。中位总生存期(median overall survival,mOS)为3.0(0.7~15.5)个月,中位无进展生存期(median progression-free survival,mPFS)为3.0(0.7~15.5)个月。在随访期间未观察到与治疗相关的严重不良事件的发生。 结论 NK/T-LAHS患者在初始诱导治疗中应用RuE-DDGP方案治疗是安全有效的。 Abstract: Objective: To investigate the clinical efficacy and safety of ruxolitinib phosphate and etoposide combined with cisplatin, dexamethasone, gemcitabine, and pegaspargase (RuE-DDGP) as an initial induction regimen for the treatment of natural killer/T-cell lymphoma-associated hemophagocytic syndrome (NK/T-LAHS). Methods: The clinical data for 11 patients with NK/T-LAHS treated with the RuE-DDGP regimen in The First Affiliated Hospital of Zhengzhou University from January 2021 to June 2022 were retrospectively analyzed for treatment efficacy, adverse effects, and prognosis. Results: Among the 11 patients, there were nine males and two females, with a median age of 30 (20–75) years. After 2 weeks of treatment, all 11 patients achieved partial remission (PR), with an overall remission rate (ORR) of 100%. After 4 weeks of treatment, 10 patients achieved PR and one was not evaluated due to death. The median overall survival (mOS) time was 3 (0.7–15.5) months, and the median progression-free survival (mPFS) time was 3 (0.7–15.5) months. No treatment-related serious adverse events were observed during the follow-up period. Conclusions: The RuE-DDGP treatment regimen is safe and effective for patients with NK/T-LAHS during initial induction therapy. -
表 1 患者的临床资料汇总
编号 性别 年龄(岁) 淋巴瘤诊断 Ann Arbor
分期(期)CA
分期(期)PINK-E
评分类型 脾大 NK细胞
活性血清EBV-DNA
治疗前/后骨髓噬血现象
治疗前/后开始治疗后持续
发热时间(天)1 男 33 NKTCL Ⅳ Ⅱ 高危组 1 是 正常 阳/阴 有/无 3 2 男 20 NKTCL Ⅳ Ⅱ 高危组 1 是 降低 阳/阴 有/有 16 3 男 64 NKTCL Ⅳ Ⅱ 高危组 1 是 降低 阳/阴 有/无 7 4 男 39 NKTCL Ⅳ Ⅲ 中危组 1 否 正常 阳/阴 有/无 7 5 男 25 NKTCL Ⅲ Ⅲ 中危组 1 否 降低 阳/阴 有/无 2 6 男 75 NKTCL Ⅳ Ⅳ 高危组 1 是 降低 阳/- 有/无 3 7 女 48 NKTCL Ⅳ Ⅳ 高危组 2 是 降低 阳/阴 有/无 3 8 男 34 NKTCL Ⅳ Ⅱ 中危组 1 是 正常 阳/阳 有/有 4 9 男 22 侵袭性NK细胞白血病 Ⅳ Ⅳ 高危组 1 是 正常 阳/阳 有/无 5 10 女 30 NKTCL Ⅳ Ⅳ 高危组 1 是 降低 阳/阳 有/− 21 11 男 24 NKTCL Ⅳ Ⅳ 高危组 1 是 正常 阳/阳 有/无 2 类型1:淋巴瘤诱导的HLH;类型2:化疗期合并的HLH;血清EBV-DNA阳性定义为>5E+02拷贝/mL,阴性定义为<5E+02拷贝/mL 表 2 应用RuE-DDGP方案治疗前后的指标变化
指标 正常范围 治疗前
中位数(范围)治疗2周后
中位数(范围)治疗4周后
中位数(范围)Neu(×109/L) 1.80~6.30 1.25(0.36~3.04) 1.72(0.03~8.60) 3.70(1.18~6.45) Hb(g/L) 130.00~175.00 89.00(67.00~128.00) 100.00(85.00~124.00) 102.00(78.00~121.00) PLT(×109/L) 125.00~350.00 39.00(4.00~113.00) 94.00(4.00~210.00) 117.00(38.00~376.00) 铁蛋白(ng/mL) 30.00~400.00 4 370.00(2 203.00~36 844.00) 3 354.00(1 636.00~13 762.00) 1 756.00(726.00~6 324.00) FIB(g/L) 2.00~4.00 1.42(0.45~3.18) 1.48(0.82~3.57) 1.82(0.86~3.01) ALT(U/L) 0~40.00 67.00(33.00~390.00) 67.00(21.00~130.00) 35.00(14.00~74.00) 甘油三酯(mmol/L) <1.70 2.95(1.10~6.57) 1.52(1.20~3.44) 1.23(0.37~2.56) sCD25(pg/mL) <6 400.00 37 816.00(3 164.00~234 790.00) - - Neu:中性粒细胞;Hb:血红蛋白;PLT:血小板;FIB:纤维蛋白原;ALT:谷丙转氨酶。由于编号10的患者在确诊后仅生存0.7个月,因此治疗4周后的数据仅为其余10例。 表 3 RuE-DDGP方案以及后续治疗方案用于NKT-LAHS患者的疗效
编号 RuE-DDGP方案
治疗2周/4周后续淋巴瘤
治疗方案淋巴瘤
化疗疗效2个
周期4个
周期1 PR/PR DDGP(6) PR CR 2 PR/PR 改良SMILE(1) - - 3 PR/PR DDGP (1) - - 4 PR/PR 改良SMILE (1) - - 5 PR/PR DDGP (1) ,PD-1 (1) CR - 6 PR/PR 无 - - 7 PR/PR DDGP(1),改良SMILE+PD-1(1) PD - 8 PR/PR P-GemOxD (1),DDGP(1) PD - 9 PR/PR DDGP(1),改良SMILE(1) PD - 10 PR/- 无 - - 11 PR/PR DDGP(3) PR - 改良SMILE(甲氨蝶呤+地塞米松+左旋门冬酰胺酶+依托泊苷+异环磷酰胺);P-GemOxD(培门冬酶+吉西他滨+奥沙利铂+地塞米松) 表 4 11例RuE-DDGP方案治疗NKT-LAHS患者的不良反应
编号 3~4级中性粒细胞减少持续时间(天) 3~4级血小板减少持续时间(天) 感染情况 3~4级肝功能不全持续时间(天) 胃肠道反应 A B A B A B 1 2 0 5 0 无 9 0 无 2 13 25 28 25 肺部感染 11 5 无 3 15 0 17 0 无 0 0 腹泻 4 4 14 6 16 无 4 0 无 5 0 0 0 0 无 0 0 恶心呕吐 6 0 0 5 0 无 0 0 无 7 0 0 11 3 无 3 0 无 8 0 0 28 20 无 0 0 无 9 13 8 3 8 肺部感染 0 0 无 10 15 - 19 - 无 0 - 无 11 11 0 3 0 无 0 0 无 A:采用RuE-DDGP方案治疗期间;B:治疗结束后随访30天期间 -
[1] 中国抗癌协会淋巴瘤专业委员会,中华医学会血液学分会淋巴细胞疾病学组,中国噬血细胞综合征专家联盟,等.淋巴瘤相关噬血细胞综合征诊治中国专家共识(2022年版)[J].中华医学杂志,2022,102(24):1794-1801. doi: 10.3760/cma.j.cn112137-20211109-02490 [2] Trottestam H, Horne A, Aricò M, et al. Chemoimmunotherapy for hemophagocytic lymphohistiocytosis: long-term results of the HLH-94 treatment protocol[J]. Blood, 2011, 118(17):4577-4584. doi: 10.1182/blood-2011-06-356261 [3] Li F, Li P, Zhang RY, et al. Identification of clinical features of lymphoma-associated hemophagocytic syndrome (LAHS): an analysis of 69 patients with hemophagocytic syndrome from a single-center in central region of China[J]. Med Oncol, 2014, 31(4):902. doi: 10.1007/s12032-014-0902-y [4] Acosta SG, Rincón MJ. Ruxolitinib as first-line therapy in secondary hemophagocytic lymphohistiocytosis and HIV infection[J]. Int J Hematol, 2020, 112(3):418-421. doi: 10.1007/s12185-020-02882-1 [5] Wang XH, Zhang L, Liu XL, et al. Efficacy and safety of a pegasparaginase-based chemotherapy regimen vs an L-asparaginase-based chemotherapy regimen for newly diagnosed advanced extranodal natural killer/T-cell lymphoma: a randomized clinical trial[J]. JAMA Oncol, 2022, 8(7):1035-1041. doi: 10.1001/jamaoncol.2022.1968 [6] Henter JI, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis[J]. Pediatr Blood Cancer, 2007, 48(2):124-131. doi: 10.1002/pbc.21039 [7] Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia[J]. Blood, 2016, 128(3):462-463. doi: 10.1182/blood-2016-06-721662 [8] Hong HM, Li YX, Lim ST, et al. A proposal for a new staging system for extranodal natural killer T-cell lymphoma: a multicenter study from China and Asia Lymphoma Study Group[J]. Leukemia, 2020, 34(8):2243-2248. doi: 10.1038/s41375-020-0740-1 [9] Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin andnon-Hodgkin lymphoma: the Lugano classification[J]. J Clin Oncol, 2014, 32(27):3059-3068. [10] Marsh RA, Allen CE, McClain KL, et al. Salvage therapy of refractory hemophagocytic lymphohistiocytosis with alemtuzumab[J]. Pediatr Blood Cancer, 2013, 60(1):101-109. doi: 10.1002/pbc.24188 [11] Humblet-Baron S, Franckaert D, Dooley J, et al. IFN-γ and CD25 drive distinct pathologic features during hemophagocytic lymphohistiocytosis[J]. J Allergy Clin Immunol, 2019, 143(6):2215-2226. doi: 10.1016/j.jaci.2018.10.068 [12] Janka GE, Lehmberg K. Hemophagocytic syndromes: an update[J]. Blood Rev, 2014, 28(4):135-142. doi: 10.1016/j.blre.2014.03.002 [13] Sano H, Kobayashi R, Tanaka J, et al. Risk factor analysis of non-Hodgkin lymphoma-associated haemophagocytic syndromes: a multicentre study[J]. Br J Haematol, 2014, 165(6):786-792. doi: 10.1111/bjh.12823 [14] Tamamyan GN, Kantarjian HM, Ning J, et al. Malignancy-associated hemophagocytic lymphohistiocytosis in adults: relation to hemophagocytosis, characteristics, and outcomes[J]. Cancer, 2016, 122(18):2857-2866. doi: 10.1002/cncr.30084 [15] Daver N, McClain K, Allen CE, et al. A consensus review on malignancy-associated hemophagocytic lymphohistiocytosis in adults[J]. Cancer, 2017, 123(17):3229-3240. doi: 10.1002/cncr.30826 [16] Bergsten E, Horne A, Aricó M, et al. Confirmed efficacy of etoposide and dexamethasone in HLH treatment: long-term results of the cooperative HLH-2004 study[J]. Blood, 2017, 130(25):2728-2738. doi: 10.1182/blood-2017-06-788349 [17] Yang L, Liu H, Xu XH, et al. Retrospective study of modified SMILE chemotherapy for advanced-stage, relapsed, or refractory extranodal natural killer (NK)/T cell lymphoma, nasal type[J]. Med Oncol, 2013, 30(4):720. doi: 10.1007/s12032-013-0720-7 [18] Han LJ, Li L, Wu JJ, et al. Clinical features and treatment of natural killer/T cell lymphoma associated with hemophagocytic syndrome: comparison with other T cell lymphoma associated with hemophagocytic syndrome[J]. Leuk Lymphoma, 2014, 55(9):2048-2055. doi: 10.3109/10428194.2013.876629 [19] Assi R, Verstovsek S, Daver N. ‘JAK-ing’ up the treatment of primary myelofibrosis: building better combination strategies[J]. Curr Opin Hematol, 2017, 24(2):115-124. [20] Maschalidi S, Sepulveda FE, Garrigue A, et al. Therapeutic effect of JAK1/2 blockade on the manifestations of hemophagocytic lymphohistiocytosis in mice[J]. Blood, 2016, 128(1):60-71. doi: 10.1182/blood-2016-02-700013 [21] Vannucchi AM, Harrison CN. Emerging treatments for classical myeloproliferative neoplasms[J]. Blood, 2017, 129(6):693-703. doi: 10.1182/blood-2016-10-695965 [22] Zhang Q, Zhao YZ, Ma HH, et al. A study of ruxolitinib response-based stratified treatment for pediatric hemophagocytic lymphohistiocytosis[J]. Blood, 2022, 139(24):3493-3504. doi: 10.1182/blood.2021014860 [23] Wang JS, Zhang R, Wu XY, et al. Ruxolitinib-combined doxorubicin-etoposide-methylprednisolone regimen as a salvage therapy for refractory/relapsed haemophagocytic lymphohistiocytosis: a single-arm, multicentre, phase 2 trial[J]. Br J Haematol, 2021, 193(4):761-768. [24] Das R, Guan P, Sprague L, et al. Janus kinase inhibition lessens inflammation and ameliorates disease in murine models of hemophagocytic lymphohistiocytosis[J]. Blood, 2016, 127(13):1666-1675. [25] Mayfield JR, Czuchlewski DR, Gale JM, et al. Integration of ruxolitinib into dose-intensified therapy targeted against a novel JAK2 F694L mutation in B-precursor acute lymphoblastic leukemia[J]. Pediatr Blood Cancer, 2017, 64(5):781-784. [26] Hu Y, Hong Y, Xu YJ, et al. Inhibition of the JAK/STAT pathway with ruxolitinib overcomes cisplatin resistance in non-small-cell lung cancer NSCLC[J]. Apoptosis, 2014, 19(11):1627-1636. doi: 10.1007/s10495-014-1030-z [27] Meyer LK, Verbist KC, Albeituni S, et al. JAK/STAT pathway inhibition sensitizes CD8 T cells to dexamethasone-induced apoptosis in hyperinflammation[J]. Blood, 2020, 136(6):657-668. doi: 10.1182/blood.2020006075 [28] Chang Y, Cui M, Fu XR, et al. Lymphoma associated hemophagocytic syndrome: a single-center retrospective study[J]. Oncol Lett, 2018, 16(1):1275-1284. [29] Schram AM, Comstock P, Campo M, et al. Haemophagocytic lymphohistiocytosis in adults: a multicentre case series over 7 years[J].Br J Haematol, 2016, 172(3):412-419. -



 下载:
下载: