Clinical study of venetoclax combined with hypomethylating drugs for the treatment of acute myeloid leukemia
-
摘要:
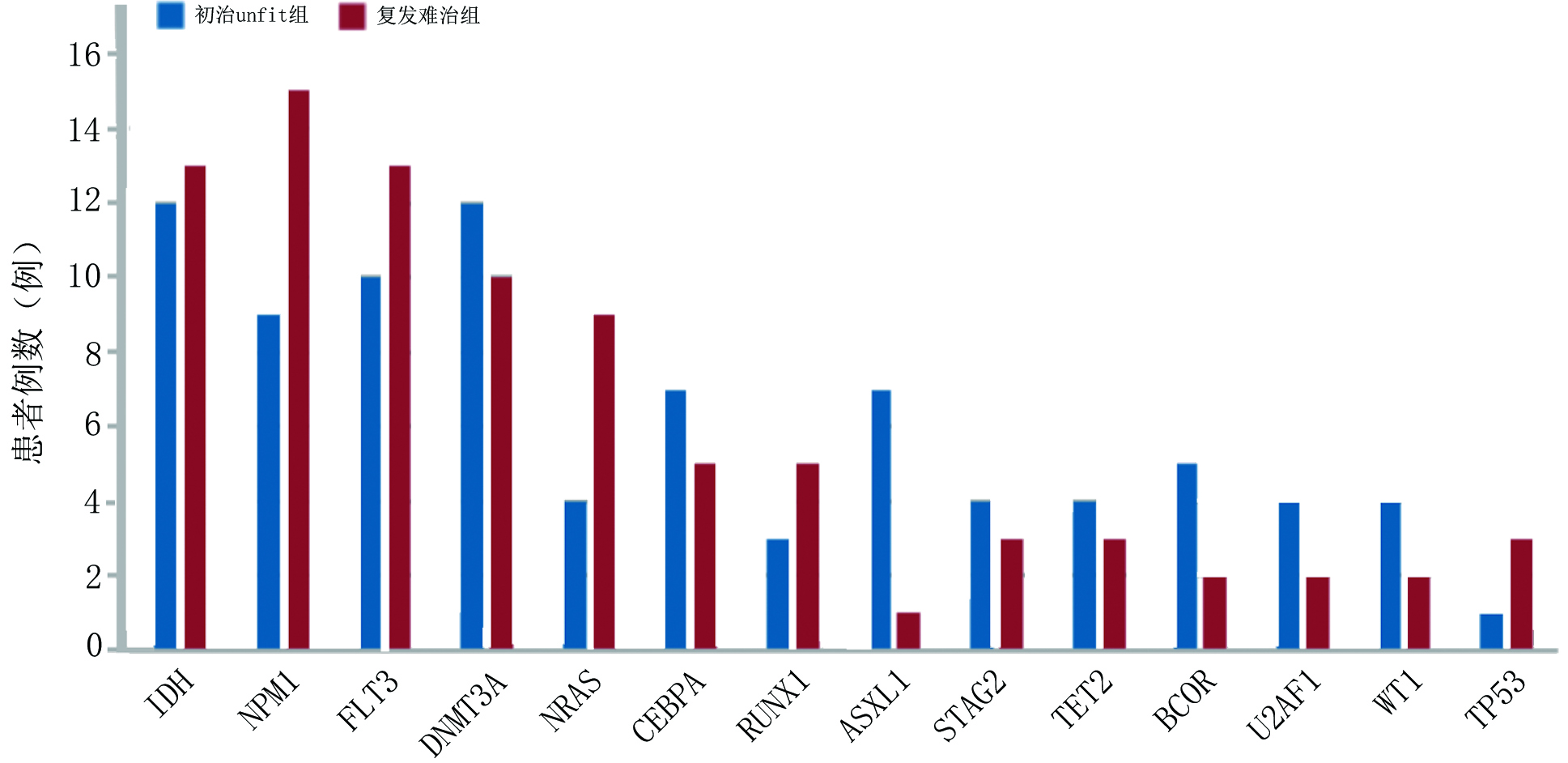
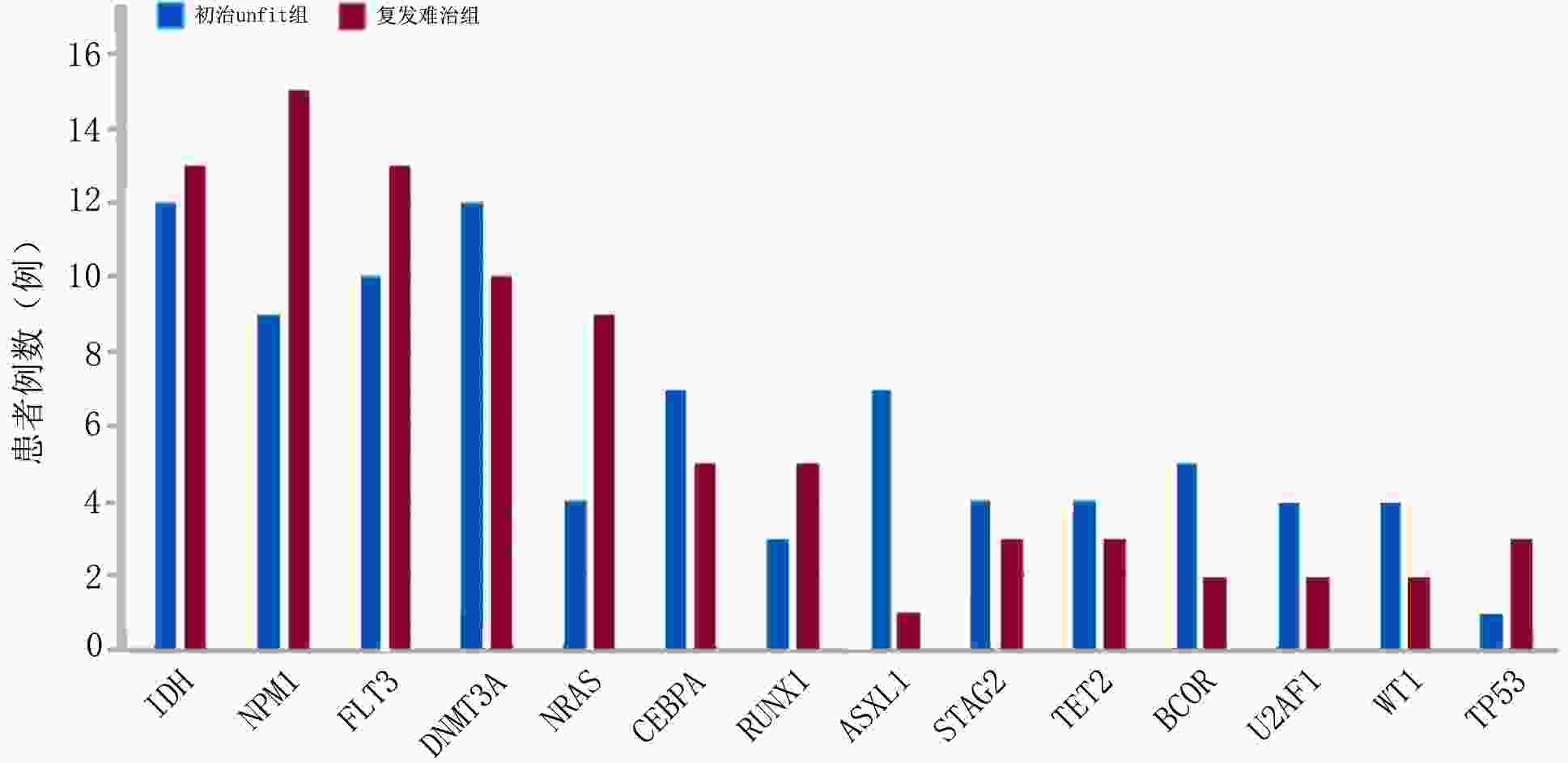
目的 探讨维奈克拉联合去甲基化药物在不适合强化诱导化疗的初治急性髓系白血病(acute myeloid leukemia,AML)及复发难治性AML(relapsed/refractory AML,R/R AML)患者中的疗效、预后及安全性。 方法 回顾性分析2020年7月至2022年10月于山东大学齐鲁医院收治的87例使用维奈克拉联合去甲基化药物治疗的AML患者,分为初治不适合强化化疗(unfit)组及复发难治组,收集患者临床资料,对两组患者的疗效、生存时间及不良反应进行分析。 结果 在疗效评估中初治unfit组中位治疗疗程为3(1~14)个,1个疗程后完全缓解(complete response,CR)率为42.86%,CR/完全缓解伴血液学不完全恢复(CR with incomplete hematologic recovery, CRi)率为77.55%, 总反应率(overall response rate,ORR)为83.67%,可检测残留病变(measurable residual disease,MRD)转阴率为34.69%;复发难治组中位治疗疗程为2(1~10)个疗程,1个疗程后CR率为23.68%, CR/CRi率为52.63%, ORR为 60.53%,MRD转阴率为31.58%。基因型与疗效的相关分析显示复发难治组中异柠檬酸脱氢酶(isocitrate dehydrogenase, IDH)突变型CR/CRi率及MRD转阴率较IDH野生型更高。在生存分析中初治unfit组中位随访时间为14个月,中位生存时间未达到,1年累计生存率为64.6%;复发难治组中位随访时间为8个月,中位生存时间为9个月,1年累计生存率为46.7%。在安全性评估中血液系统不良反应发生率为100%; 非血液系统不良反应发生率为97.7%,2.3%患者出现肿瘤溶解综合征(tumor lysis syndrome,TLS)。 结论 维奈克拉联合去甲基化药物在初治unfit的AML及复发难治性AML患者中疗效肯定,几乎所有接受该方案治疗的患者均会出现不良反应,但大多数患者可耐受,治疗过程中需加强对TLS监测。 -
关键词:
- 维奈克拉 /
- 去甲基化药物 /
- 复发难治性急性髓系白血病
Abstract: Objective: To evaluate the efficacy, prognosis, and safety of venetoclax combined with hypomethylating drugs in patients with untreated acute myeloid leukemia (AML) who were not suitable for intensive therapy and in patients with relapsed/refractory AML (R/R AML). Methods: A retrospective analysis was performed for 87 patients with AML who were treated with venetoclax combined with hypomethylating agents at Qilu Hospital of Shandong University from July 2020 to October 2022. They were assigned into the untreated unfit group and the relapsed/refractory group. The clinical data of the patients were collected, and the efficacy, survival and adverse events were analyzed. Results: Efficacy evaluation: The median course for the untreated unfit group was 3 (range: 1–14), and the proportions of patients achieving complete response (CR), CR/CR with incomplete hematologic recovery (CR/CRi), overall response rate (ORR), and measurable residual disease (MRD) negative rate was 42.86%, 77.55%, 83.67% and 34.69%, respectively. The median course for the relapsed/refractory group was 2 (range: 1–10), and the proportion of patients achieving CR, CR/CRi, ORR and MRD negative was 23.68%, 52.63%, 60.53% and 31.58%, respectively. The correlation analysis between genotype and efficacy showed that patients with isocitrate dehydrogenase (IDH) mutations had a higher CR/CRi rate and MRD negative rate as compared to patients with IDH wild type. Survival analysis: The median follow-up time of the untreated unfit group was 14 months, the median survival time was not reached, and the one year cumulative survival rate was 64.6%. The median follow-up time of the relapsed/refractory group was 8 months, the median survival time was 9 months, and the one year cumulative survival rate was 46.7%. Safety evaluation: The incidence of hematological and non-hematological adverse events was 100.00% and 97.70%, respectively. Furthermore, tumor lysis syndrome (TLS) occurred in 2.3% patients. Conclusions: Venetoclax combined with hypomethylating drugs is effective in patients with untreated unfit AML and R/R AML. Almost all the patients experience adverse events; however, they are tolerable for most patients. Monitoring for TLS should be strengthened during treatment. -
表 1 患者基线特征
例(%) 临床特征 初治unfit组(n=49) 复发难治组(n=38) 总计 年龄(岁) <65 20(40.82) 29(76.32) 49(56.32) ≥65 29(59.18) 9(23.68) 38(43.68) 性别 男 20(40.82) 15(39.47) 35(40.23) 女 29(59.18) 23(60.53) 52(59.77) AML类型 原发性 42(85.71) 35(92.11) 77(88.51) 继发性* 7(14.29) 3(7.89) 10(11.49) FAB分型 M0 3(6.12) 3(7.89) 6(6.90) M1 1(2.04) 0 (0) 1(1.15) M2 5(10.20) 2(5.26) 7(8.05) M4 5(10.20) 5(13.17) 10(11.49) M5 31(63.27) 22(57.89) 53(60.92) 不确定 4(8.17) 6(15.79) 10(11.49) 外周血白细胞计数(×109/L) <4 21(42.86) 20(52.63) 41(47.12) 4~10 12(24.49) 8(21.05) 20(22.99) >10 16(32.65) 10(26.32) 26(29.89) 乳酸脱氢酶(U/L) ≤230 12(24.49) 19(50.00) 31(35.63) >230 37(75.51) 19(50.00) 56(64.37) 骨髓原始细胞(%) <30 9(18.37) 16(42.10) 25(28.74) 30~50 11(22.45) 6(15.79) 17(19.54) >50 28(57.14) 16(42.11) 44(50.57) 不详 1(2.04) 0 1(1.15) ELN预后危险度分层 预后良好 8(16.33) 9(23.69) 17(19.54) 预后中等 21(42.86) 14(36.84) 35(40.23) 预后不良 20(40.82) 15(39.47) 35(40.23) HMA 阿扎胞苷 47(95.92) 29(76.31) 76(87.36) 地西他滨 2(4.08) 5(13.16) 7(8.04) 阿扎胞苷/地西他滨 0 4(10.53) 4(4.60) 联合使用抗真菌药 泊沙康唑 9(18.37) 2(5.26) 11(12.64) 伏立康唑 35(71.43) 29(76.32) 64(73.56) *:继发性AML包括MDS/MPN转化、治疗相关性AML 表 2 复发组及难治组患者1个周期后疗效比较
项目 复发组(n=26) 难治组(n=12) P CR 5(19.23) 4(33.33) 0.423 CRi 13(50.00) 7(58.33) 0.734 ORR 15(57.69) 8(66.67) 0.728 MRD转阴 7(26.92) 5(41.67) 0.460 ()内单位为% 表 3 基因突变与疗效的相关性分析
基因型 CR/CRi(例) P ORR(例) P MRD转阴(例) P 初治unfit组 IDH 0.081 0.190 0.079 突变型 12 12 6 野生型 26 29 11 NPM1 0.645 >0.999 0.170 突变型 8 8 6 野生型 30 33 11 FLT3 0.527 0.277 0.306 突变型 9 10 6 野生型 29 31 11 复发难治组 IDH 0.043 0.176 0.009 突变型 10 10 8 野生型 10 13 4 NPM1 >0.999 >0.999 >0.999 突变型 8 9 5 野生型 12 14 7 FLT3 0.734 0.728 >0.999 突变型 6 7 4 野生型 14 16 8 表 4 87例患者非血液学不良反应分析
项目 例(%) 感染 76(87.36) 肺部感染 75(86.21) 皮肤软组织感染 8(9.20) 尿路感染 1(1.15) 呕吐 33(37.93) 便秘 40(45.98) 腹泻 17(19.54) 低钾血症 24(27.59) 心律失常 3(3.45) 皮疹 5(5.75) 肝功能异常 25(28.74) TLS 2(2.30) 其他 癫痫 1(1.15) 下肢麻木 1(1.15) 头痛 1(1.15) 表 5 87例患者血液学不良反应分析
例(%) 项目 分级1~5级 分级≥3级 白细胞减少 87(100) 80(91.95) 中性粒细胞减少 85(97.70) 81(93.10) 发热伴中性粒细胞减少 56(64.37) 56(64.37) 贫血 86(98.85) 71(81.61) 血小板减少 79(90.80) 71(81.61) -
[1] Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts[J]. Blood, 2015, 126(3):291-299. doi: 10.1182/blood-2015-01-621664 [2] DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia[J]. Blood, 2019, 133(1):7-17. doi: 10.1182/blood-2018-08-868752 [3] DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia[J]. N Engl J Med, 2020, 383(7):617-629. doi: 10.1056/NEJMoa2012971 [4] Konopleva M, Pollyea DA, Potluri J, et al. Efficacy and biological correlates of response in a phaseⅡstudy of venetoclax monotherapy in patients with acute myelogenous leukemia[J]. Cancer Discov, 2016, 6(10):1106-1117. doi: 10.1158/2159-8290.CD-16-0313 [5] Palmieri R, Othus M, Halpern AB, et al. Accuracy of SIE/SIES/GITMO consensus criteria for unfitness to predict early mortality after intensive chemotherapy in adults with AML or other high-grade myeloid neoplasm[J]. J Clin Oncol, 2020, 38(35):4163-4174. [6] Zeidan AM, Fenaux P, Gobbi M, et al. Prospective comparison of outcomes with azacitidine and decitabine in patients with AML ineligible for intensive chemotherapy[J]. Blood, 2022, 140(3):285-289. doi: 10.1182/blood.2022015832 [7] Pollyea DA, Pratz K, Letai A, et al. Venetoclax with azacitidine or decitabine in patients with newly diagnosed acute myeloid leukemia: long term follow-up from a phase 1b study[J]. Am J Hematol, 2021, 96(2):208-217. doi: 10.1002/ajh.26039 [8] Richard-Carpentier G, DiNardo CD. Venetoclax for the treatment of newly diagnosed acute myeloid leukemia in patients who are ineligible for intensive chemotherapy[J]. Ther Adv Hematol, 2019, 10, 34(8):2040620719882822. [9] Chen Y, Cao J, Ye YZ, et al. Hypomethylating agents combined with low-dose chemotherapy for elderly patients with acute myeloid leukaemia unfit for intensive chemotherapy: a real-world clinical experience[J]. J Chemother, 2022, 28(13):1-8. [10] Aldoss I, Yang DY, Aribi A, et al. Efficacy of the combination of venetoclax and hypomethylating agents in relapsed/refractory acute myeloid leukemia[J]. Haematologica, 2018, 103(9):e404-e407. doi: 10.3324/haematol.2018.188094 [11] Wang YW, Tsai CH, Lin CC, et al. Cytogenetics and mutations could predict outcome in relapsed and refractory acute myeloid leukemia patients receiving BCL-2 inhibitor venetoclax[J]. Ann Hematol, 2020, 99(3):501-511. doi: 10.1007/s00277-020-03911-z [12] DiNardo CD, Maiti A, Rausch CR, et al. 10-day decitabine with venetoclax for newly diagnosed intensive chemotherapy ineligible, and relapsed or refractory acute myeloid leukaemia: a single-centre, phase 2 trial[J]. Lancet Haematol, 2020, 7(10):e724-e736. doi: 10.1016/S2352-3026(20)30210-6 [13] Bewersdorf JP, Giri S, Wang R, et al. Venetoclax as monotherapy and in combination with hypomethylating agents or low dose cytarabine in relapsed and treatment refractory acute myeloid leukemia: a systematic review and meta-analysis[J]. Haematologica, 2020, 105(11):2659-2663. doi: 10.3324/haematol.2019.242826 [14] Maiti A, DiNardo CD, Qiao W, et al. Ten-day decitabine with venetoclax versus intensive chemotherapy in relapsed or refractory acute myeloid leukemia: a propensity score-matched analysis[J]. Cancer, 2021, 127(22):4213-4220. doi: 10.1002/cncr.33814 [15] 王馨,鲍协炳,张剑,等.Bcl-2抑制剂维奈克拉联合用药治疗IDH1/2突变阳性急性髓系白血病的疗效及安全性分析[J].中华血液学杂志,2022,43(8):691-694. [16] Bewersdorf JP, Abdel-Wahab O. Translating recent advances in the pathogenesis of acute myeloid leukemia to the clinic[J]. Genes Dev, 2022, 36(5):259-277. [17] DiNardo CD, Wei AH. How I treat acute myeloid leukemia in the era of new drugs[J]. Blood, 2020, 135(2):85-96. doi: 10.1182/blood.2019001239 -



 下载:
下载: