Mechanism of Inhibitory Effect of Ouabain on the Proliferation and Invasion of Human Colorectal Cancer Multidrug-Resistant Cells
-
摘要:
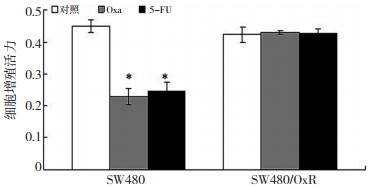
目的 探讨结直肠癌耐药细胞增殖及侵袭力与Na+, K+-ATP酶活性及其β1亚单位和P糖蛋白(P-gp)表达的关系, 及哇巴因增加化疗敏感性的可能机制。 方法 以人结直肠癌亲本细胞(SW480)和耐奥沙利铂细胞(SW480/OxR)为研究对象, 采用MTS法、Transwell小室、生化酶学、实时定量PCR(Real time quantitative, RT-PCR)及流式细胞技术等方法比较SW480细胞与SW480/OxR细胞的增殖及侵袭力、Na+, K+-ATP酶活性及其β1亚单位和P-gp表达的差异, 观察哇巴因对SW480/OxR细胞上述指标的影响。 结果 与SW480细胞比较, SW480/OxR细胞增殖活力无明显变化(P > 0.05), 而细胞侵袭力却显著增加, Na+, K+-ATP酶活性下降, β1亚单位表达下调, P-gp表达上调(P均 < 0.01);哇巴因能显著抑制SW480/OxR细胞增殖活力, 减弱其侵袭力, 下调SW480/OxR细胞P-gp蛋白表达, 上调SW480/OxR细胞β1亚单位蛋白表达, 增加SW480/OxR细胞Na+, K+-ATP酶活性(P均 < 0.01)。 结论 Na+, K+-ATP酶活性下降可能源于β1亚单位表达下调, 并参与结直肠癌的耐药; 哇巴因能部分逆转结直肠癌耐药细胞MDR, 可能与增加Na+, K+-ATP酶活性及下调P-gp表达有关。 Abstract:Objective This work aimed to explore the effects of Na+/K+-ATPase activity and the expression of itsβ1-subunit and P-glycoprotein(P-gp) on the proliferation and invasion of human colorectal cancer parental cells(SW480) and oxaliplatin-resistant cells(SW480/OxR).The molecular mechanisms of ouabain for reversing the multidrug resistance(MDR) of human colorectal cancer oxaliplatin-resistant cells were also examined. Methods SW480 and SW480/OxR cells derived from the same patient were treated with or without ouabain at the physiological concentration of 1 nM.The SW480 and SW480/OxR cell proliferation capacity was assessed by the MTS assay.The invasion capacity was deteremined using a Transwell chamber.The Na+/K+-ATPase activity was measured by biochemical and enzymological techniques.The expression of theβ1-subunit and P-gp of Na+/K+-ATPase was determined by real-time quantitative PCR, Western blotting, and flow cytometry. Results The capacity of invasion significantly increased in the SW480/OxR cells compared with the SW480 cells(P < 0.01).There was no difference between the SW480 and SW480/OxR cell proliferation capacities(P > 0.05).The Na +/K+-ATPase activity significantly decreased in SW480/OxR cells compared with SW480 cells(P < 0.01).The expression of the Na +/K+-ATPaseβ1-subunit in mRNA and protein levels was lower in SW480/OxR cells than in SW480 cells(P < 0.01).However, the expression of P-gp in mRNA and protein levels was higher in SW480/OxR cells than in SW480 cells(P < 0.01).Interestingly, ouabain at the physiological concentration of 1 nM significantly enhanced the activity of Na +/K+-ATPase in SW480/OxR cells(P < 0.05).The expression of theβ1-subunit was also upregulated and that of P-gp was downregulated at the protein level, thereby inducing a decrease in the capacity of SW480/OxR cell growth inhibition and invasion.Nevertheless, after ouabain treatment for 48 h, the Na +/K+-ATPase activity andβ1-subunit protein expression level in SW480/OxR cells were still lower than those in SW480 cells(P < 0.01). Conclusion Decreased Na +/K+-ATPase activity can be attributed to the downregulation of Na+/K+-ATPaseβ1-subunit expression and may cause the MDR of human colorectal cancer cells.Ouabain could partly reverse the MDR of such cells, which can be attributed to increased Na+/K+-ATPase activity and P-gp expression downregulation. -
Key words:
- Colorectal cancer /
- Na+/K+-ATPase /
- P-glycoprotein /
- Multidrug resistance of chemotherapy /
- Ouabain
-
表 1 荧光实时定量PCR引物序列
Table 1. Primer sequence used in real-time quantitative PCR

-
[1] Geering K. The functional role of beta subunits in oligomeric P-type ATPase[J]. J Bioenerg Biomembr, 2001, 33(5): 425-438. doi: 10.1023/A:1010623724749 [2] Kitamura N, Ikekita M, Sato T, et al. Mouse Na+/K+-ATPaseβ1-subunit has a K+-dependent cell adhesion activity forβ-GlcNActerminating glycans[J]. Proc Natl Acad Sci U S A, 2005, 102(8): 2796-2801. doi: 10.1073/pnas.0409344102 [3] Shoshani L, Contreras RG, Roldan ML, et al. The polarized expression of Na+, K+-ATPase in epithelia depends on the association betweenβ-subunits located in neighboring cells[J]. Mol Biol Cell, 2005, 16(3): 1071-1081. doi: 10.1091/mbc.e04-03-0267 [4] Rajasekaran SA, Huynh TP, Wolle DG, et al. Na, K-ATPase Subunits as Markers for Epithelial-Mesenchymal Transition in Cancer and Fibrosis[J]. Mol Cancer Ther, 2010, 9(6): 1515-1524. doi: 10.1158/1535-7163.MCT-09-0832 [5] Inge LJ, Rajasekaran SA, Yoshimoto K, et al. Evidence for a potential tumor suppressor role for the Na, K-ATPaseβ1-subunit[J]. Histol Histopathol, 2008, 23(4): 459-467. http://digitum.um.es/jspui/bitstream/10201/29794/1/Evidence%20for%20a%20potential%20tumor%20suppressor%20role%20for%20the%20Na,K-ATPase%20B1-subunit.pdf [6] Espineda C, Seligson DB, Ball Jr. JW, et al. Analysis of the Na, K-ATPaseα- andβ-subunit expression profiles of bladder cancer using tissue microarrays[J], Cancer, 2003, 97(8): 1859-1868. [7] Espineda C, Chang JH, Twis J, et al. Repression of Na, K-ATPaseβ1-subunit by the transcription factor Snail in carcinoma[J]. Mo Biol Cell, 2004, 15(2): 1364-1373. http://www.molbiolcell.org/cgi/reprint/15/3/1364.pdf [8] Aperia A. New roles for an old enzyme: Na, K-ATPase emerges as an interesting drug target[J]. Intern Med, 2007, 261(1): 44-52. http://www.onacademic.com/detail/journal_1000034526975610_e688.html [9] Liu J, TianJ, Haas M, et al. Ouabain interaction with cardiac Na+/ K+-ATPase initiates signal cascades independent of changes in intracellular Na+ and Ca2+ concentrations[J]. J Biol Chem, 2000, 275 (36): 27838-27844. doi: 10.1074/jbc.M002950200 [10] Yang AD, Fan F, Camp ER, et al. Chronic Oxaliplatin Resistance Induces Epithelial-to-Mesenchymal Transition in Colorectal Cancer Cell Lines[J]. Clin Cancer Res, 2006, 12(14): 4147-4153. doi: 10.1158/1078-0432.CCR-06-0038 [11] 张贵海, 商黔惠, 姜黔峰, 等. 血管紧张素Ⅱ对正常和高血压大鼠主动脉平滑肌细胞腺苷三磷酸酶的影响[J]. 中国动脉硬化杂志, 2010, 18 (4): 260-264. https://www.cnki.com.cn/Article/CJFDTOTAL-KDYZ201004004.htm [12] Borst P, Elferink RO. Mammalian ABC transporter in health and disease[J]. Annu Rev Biochem, 2002, 71: 537-592. doi: 10.1146/annurev.biochem.71.102301.093055 [13] Dallas NA, Ling X, Fan F, et al. Chemoresistant Colorectal Cancer Cells, the Cancer Stem Cell Phenotype, and Increased Sensitivity to Insulin-like Growth Factor-I Receptor Inhibition[J]. Cancer Res, 2009, 69(5): 1951-1957. doi: 10.1158/0008-5472.CAN-08-2023 -




 下载:
下载: