Changes in Dendritic Cells of Peripheral Blood in 74 Lung Cancer Patients before and after GVAX Treatment and Their Clinical Significance
-
摘要:
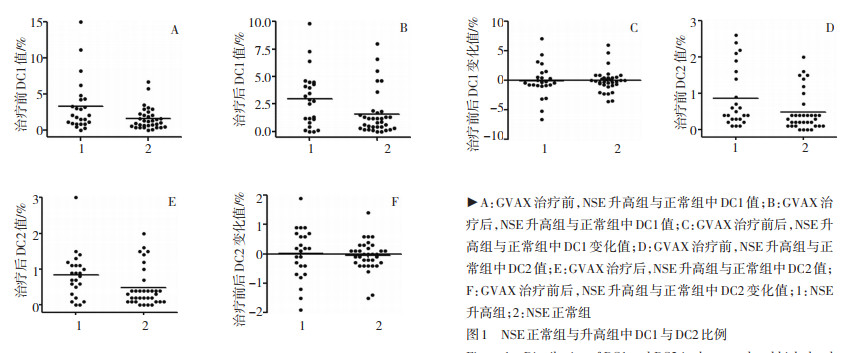
目的 探讨粒-巨噬细胞集落刺激因子(granulocyte-macrophage colony stimulating factor,GM-CSF)基因修饰的肿瘤细胞疫苗治疗前后肺癌患者外周血中DC两个不同亚群(DC1,DC2)的比例、治疗前后比例变化与临床病理特征的相关性及对生存的影响。 方法 对74例接受GM-CSF基因修饰的肿瘤细胞疫苗(GM- CSF modified tumor cell vaccine,GVAX)治疗的肺癌患者,采用流式细胞技术检测患者治疗前后外周血DC及淋巴细胞亚群的比例,分析治疗前后DC比例的变化、治疗前后DC比例与治疗前血清标志物及免疫细胞的关系、治疗前后DC比例对肺癌患者生存的影响。 结果 接受GVAX疫苗治疗后外周血DC1与DC2比例无明显变化(PDC1=0.786,PDC2=0.779);神经烯醇化酶(NSE)水平升高组中的DC亚群比例高于NSE水平正常组;治疗后DC2比例与治疗前Treg呈负相关;对于早期肺癌患者,治疗后DC2比例低于均值者的生存时间比高于均值者的生存时间明显延长。 结论 治疗后DC2比例可作为早期患者GVAX疫苗疗效及判断预后的免疫指标,其对预后的影响可能与患者外周血Treg比例有一定的相关性。 -
关键词:
- 肺癌 /
- 树突状细胞 /
- 调节性T细胞 /
- 粒-巨噬细胞集落刺激因子 /
- 预后
Abstract:Objective Dendritic cells (DC) can be divided into two subgroups based on the markers and functions: myeloid-DC (DC1) and plasmacytoid-DC (DC2). This study evaluated the clinical significance of the proportion of DC subgroups in the peripheral blood of lung cancer patients before and after GVAX vaccineation. Methods Seventy-four patients with lung cancer were enrolled in the study. Flow cytometry was used to detect the proportion of DCs and lymphocyte subgroups before and after GVAX vaccination. The correlation of DCs with serum markers and immune cells was tested. The Kaplan-Meier was used to analyze the correlations between DCs and survival of patients. Results There was no significant change in the proportion of DCs after GVAX vaccination. The proportion of DCs was higher in the group with elevated levels of neuron-specific enolase (NSE) than in normal controls. A negative correlation was observed between the proportion of DC2 post-GVAX vaccination and the proportion of Treg pre-GVAX vaccination. For patients with early lung cancer, those with a proportion of DC2 below the mean value after vaccination had better overall survival compared with those with a proportion of DC2 greater than the mean value. Conclusion A low proportion of DC2 after GVAX vaccination is a predictor of better prognosis for patients who received GVAX vaccination. DC2 proportion post-GVAX vaccination may have a correlation with the proportion of Treg in the peripheral blood pre-GVAX vaccination. -
Key words:
- Lung cancer /
- Dendritic cells (DCs) /
- Regulatory T cell /
- GM-CSF /
- Prognosis
-
表 1 GVAX治疗前后肺癌患者外周血DC细胞分布
x±s,% Table 1. Distribution of DCs before and after GVAX vaccination in the different groups
x±s, % 
表 2 DC与GVAX治疗前实验室检测指标相关性
Table 2. Correlation between DC and indices tested in the laboratory before GVAX treatment

-
[1] 邹小农. 中国肺癌流行病学[J]. 中华肿瘤防治杂志, 2007, 14(12): 881-883. doi: 10.3969/j.issn.1673-5269.2007.12.001 [2] Nemunaitis J, Jahan T, Ross H, et al. Phase 1/2 trial of autologous tumor mixed with an allogeneic GVAX vaccine in advanced-stage non-small-cell lung cancer[J]. Cancer Gene Ther, 2006, 13(6): 555-562. doi: 10.1038/sj.cgt.7700922 [3] Huang AY, Bruce AT, Pardoll DM, et al. In vivo cross-priming of MHC class I-restricted antigens requires TAP transporter[J]. Immunity, 1996, 4(4): 349-355. doi: 10.1016/S1074-7613(00)80248-4 [4] Huang AY, Golumbek P, Ahmdzadeh M, et al. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens[J]. Science, 1994, 264(5161): 961-965. doi: 10.1126/science.7513904 [5] 杜春娟, 于津浦, 李慧, 等. GM- CSF修饰肿瘤细胞疫苗治疗前后肺癌患者Treg的变化及其临床意义[J]. 中国肿瘤生物治疗, 2011.18(5): 473-479. doi: 10.3872/j.issn.1007-385X.2011.05.002 [6] Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. morphology, quantitation, tissue distribution[J]. J Exp Med, 1973, 137(5): 1142-1162. doi: 10.1084/jem.137.5.1142 [7] Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice[J]. J Immunol, 2007, 178(1): 5-25. http://www.ncbi.nlm.nih.gov/pubmed/17182535 [8] Hege KM, Jooss K, Pardoll D. GM-CSF gene-modifed cancer cell immunotherapies: of mice and men[J]. Int Rev Immunol, 2006, 25 (5-6): 321-352. doi: 10.1080/08830180600992498 [9] Small EJ, Sacks N, Nemunaitis J, et al. Granulocyte-macrophage colony-stimulating factor-secreting allogeneic cellular immunotherapy for hormone-refractory prostate cancer[J]. Clin Cancer Res, 2007, 13(13): 3883-3891. doi: 10.1158/1078-0432.CCR-06-2937 [10] Jaffee EM, Hruban RH, Biedrzycki B, et al. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: A phase I trial of safety and immune activation[J]. J Clin Oncol, 2001, 19(1): 145-156. doi: 10.1200/JCO.2001.19.1.145 [11] Luiten RM, Kueter EW, Mooi W, Gallee MP, et al. Immunogenicity, including vitiligo, and feasibility of vaccination with autologous GM-CSF-transduced tumor cells in metastatic melanoma patients [J]. J Clin Oncol, 2005, 23(35): 8978-8991. doi: 10.1200/JCO.2005.01.6816 [12] 康红刚, 王运良, 于津浦, 等. GM-CSF修饰的异体肿瘤细胞疫苗治疗晚期肿瘤的Ⅰ期临床试验[J]. 中国医药生物技术, 2008, 3(6)∶420-424. doi: 10.3969/j.issn.1673-713X.2008.06.006 [13] Simons JW, Carducci MA, Mikhak B, et al. Phase I/Ⅱ trial of an allogeneic cellular immunotherapy in hormone-naïve prostate cancer [J]. Clin Cancer Res 2006, 12(11Pt1): 3394-3401. http://pdfs.semanticscholar.org/759e/3cd4a2b3cd993a06ccb91697563c92d9be14.pdf [14] Sakaguchi S, Sakaguchi N, Asano M, et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha chains(CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune disease[J]. J Immunol, 1995, 155 (3): 1151-1164. http://www.ncbi.nlm.nih.gov/pubmed/7636184 [15] Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival[J]. Nat Med, 2004, 10(9): 942-949. doi: 10.1038/nm1093 [16] ZouW. Regulatory T cells, tumour immunity and immunotherapy [J]. Nat Rev Immunol, 2006, 6(4): 295-307. doi: 10.1038/nri1806 [17] Moseman EA, Liang X, Dawson AJ, et al. Human plasmacytoid dendritic cells activated by CpG oligodeoxy nucleotides induce the generation of CD4+CD25+regulatory T cells[J]. J Immunol, 2004, 173 (7): 4433-4442. doi: 10.4049/jimmunol.173.7.4433 [18] Simpson TR, Quezada SA, Allison JP. Regulation of CD4 T cell activation and effect or function by inducible costimulator(ICOS)[J]. Curr Opin Immunol, 2010, 22(3): 326-332. doi: 10.1016/j.coi.2010.01.001 [19] Ito T, Hanabuchi S, Wang YH, et al. Two functional subsets of Foxp3+ regulatory T cells in human thymus and periphery[J]. Immunity, 2008, 28(6): 870-880. doi: 10.1016/j.immuni.2008.03.018 [20] Cao X, Cai SF, Fehniger TA, et al. Granzyme B and perforin are important for regulatory T cell2 mediated suppression of tumor clearance[J]. Immunity, 2007, 27(4): 635-646. doi: 10.1016/j.immuni.2007.08.014 [21] Gondek DC, Lu LF, Quezada SA, et al. Cutting edge: contact-mediated suppression by CD4 + CD25 + regulatory cells involves a granzyme B-dependent, perforin-independent mechanism[J]. J Immunol, 2005, 174(4): 1783-1786. doi: 10.4049/jimmunol.174.4.1783 -




 下载:
下载: