Clinical Efficacy of Cytokine-induced Killer Cells for 87 Patients with Non-Small Cell Lung Cancer
-
摘要:
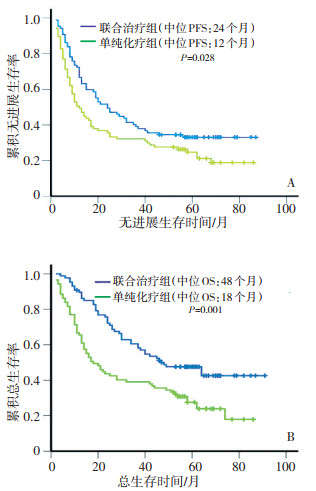
目的 评价细胞因子诱导的杀伤细胞(cytokine-induced killer cells,CIK)联合化疗治疗非小细胞肺癌(non-small cell lung cancer,NSCLC)的临床疗效。 方法 收集天津医科大学附属肿瘤医院2003年1月至2008年3月接受CIK细胞联合化疗治疗的87例NSCLC患者作为联合治疗组,接受单纯化疗的87例NSCLC患者作为对照组。Ⅰ~ⅢA期为早期,Ⅳ期为晚期。配对因素包括性别、年龄、吸烟情况、病理类型、KPS评分、临床分期、是否手术、乳酸脱氢酶(lactate dehydrogenase,LDH)、血小板、血红蛋白、治疗情况等。观察终点为无进展生存期(progression-free survival,PFS)和总生存期(overall survival,OS)。对于未取得中位OS或PFS组用平均OS或PFS表示。 结果 联合治疗组与单纯化疗组2年PFS率分别为47%、36%(P < 0.05),2年OS率分别为71%、43%(P < 0.001)。两组患者中位PFS分别为24、12个月(P < 0.05),中位OS分别为48、18个月(P=0.001)。早期患者中联合治疗组与单纯化疗组2年PFS率、中位PFS差异无明显统计学意义(74% vs. 58%,P=0.138;57个月vs. 45个月,P=0.093),联合治疗组2年OS率及中位OS明显高于单纯化疗组(92% vs. 72%,P < 0.05;73个月vs. 53个月,P < 0.05)。晚期患者中联合治疗组与单纯化疗组2年PFS率分别为13%、5%(P < 0.001),2年OS率分别为42%、3%(P < 0.001),两组患者中位PFS分别为13、6个月(P=0.001),中位OS分别为24、10个月(P=0.001)。多因素分析显示临床分期及CIK治疗周期数是联合治疗组肺癌患者的独立预后因素。 结论 CIK细胞联合化疗能够延长肺癌患者的总体生存时间,并延长晚期患者的无进展生存时间,显著改善肺癌患者预后。CIK细胞治疗多于7个周期者疗效更好。 -
关键词:
- 细胞因子诱导的杀伤细胞 /
- 非小细胞肺癌 /
- 过继性免疫细胞治疗 /
- 预后
Abstract:Objective To evaluate the clinical efficacy of cytokine-induced killer cells (CIK) combined with chemotherapy in treating patients with non-small cell lung cancer (NSCLC). Methods All NSCLC patients involved in this study were admitted and treated at Tianjin Medical University Cancer Institute and Hospital from January 2003 to March 2008. Patients received autologous CIK cells combined with chemotherapy (combined group, Group A) or chemotherapy alone (simple chemotherapy group, Group B). Patients in the two groups were matched for sex and age, smoking history, Karnofsky performance status, pathological type, clinical stage, LDH, platelets, hemoglobin, etc. Progression-free survival (PFS) and overall survival (OS) were evaluated. Results Results: The 2-year PFS and OS were 47 % and 36 %, respectively, in Group A (P < 0.05), and 71 % and 43 %, respectively, in Group B (P < 0.001). The median PFS and OS were 24 and 12 months, respectively in Group A (P < 0.05) and 48 and 18 months, respectively, in Group B (P < 0.001). There were no statistical differences in the distributions of PFS or median PFS of the patients with early stage disease between the two groups. Nevertheless, there were significant differences in the 2-year OS and median OS between the two groups (92 % vs. 72 %, P < 0.05; 73 months vs. 53 months, P < 0.05). However, the 2-year PFS and OS of advanced NSCLC patients were significantly higher in Group A than in Group B (13 % vs. 5 %, P < 0.001; and 42 % vs. 3 %, P < 0.001). The median PFS and OS of patients with advanced NSCLC were also obviously longer in Group A than in Group B (13 months vs. 6 months, P = 0.001; and 24 months vs. 10 months, P = 0.001, respectively). Multivariate analysis revealed that the cycles of CIK cellular immunotherapy were significantly correlated with longer PFS time (HR = 0.178, 95 % CI: 0.067 - 0.473, P = 0.001) and OS time (HR = 0.141, 95 % CI: 0.054 - 0.367, P < 0.001). The optimal cut-point (for grouping) of the cycles was 7 cycles. Conclusion CIK cell immunotherapy combined with chemotherapy is associated with improved prognosis for NSCLC patients. Greater number of CIK treatment cycles seems to produce greater benefit for patients. -
表 1 两组NSCLC患者临床病理特征比较
例 Table 1. Clinical characteristics of patients in the CIK group and the control group

-
[1] Jemal A, Siegel R, Ward E, et al. Cancer statistics[J]. CA Cancer J Clin, 2008, 58(2): 71-96. doi: 10.3322/CA.2007.0010 [2] Schmidt-Wolf IG, Negrin RS, Kiem HP, et al. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity[J]. J Exp Med, 1991, 174 (1): 139-149. doi: 10.1084/jem.174.1.139 [3] Li H, Wang C, Yu J, et al. Dendritic cell-activated cytokine-induced killer cells enhance the anti-tumor effect of chemotherapy on non-small cell lung cancer in patients after surgery[J]. Cytotherapy, 2009, 11(8): 1-8. http://www.sciencedirect.com/science/article/pii/S146532490970355X [4] Hammerschmidt S, Wirtz H. Lung Cancer: Current Diagnosis and Treatment[J]. Dtsch Arztebl Int, 2009, 106(49): 819-820. http://data.aerzteblatt.org/pdf/DI/106/49/m809.pdf [5] Elgert KD, Alleva DG, Mullins DW. Tumor-induced immune dysfunction: the macrophage connection[J]. J Leukoc Bio, 1998, 64(3): 275-290. doi: 10.1002/jlb.64.3.275 [6] Gritzapis AD, Dimitroulopoulos D, Paraskevas E, et al. Large scale expansion of CD3(+)CD56(+) lymphocytes capable of lysing autologous tumor cells with cyt-okine rich supernatants[J]. Cancer Immunol Immunother, 2002, 51(8): 440-448. doi: 10.1007/s00262-002-0298-y [7] Laderach D, MovassaghM, Johns om A, et al. 4-1 BB costimulation enhances human CD8 (+) T cell priming by augmenting the proliferation and survival of ef-fector CD8 (+) T cells[J]. Int Immunol, 2002, 14(10): 1155-1167. doi: 10.1093/intimm/dxf080 [8] Hoyle C, Bangs CD, Chang P, et al. Expansion of Philadelphia chromosome-neg-ative CD3+CD56+cytotoxic cells from chronic myeloid leukemia patients: in vitro and in vivo efficacy in seve recombined immuno-deciency disease mice[J]. Blood, 1998, 92(9): 3318-3327. doi: 10.1182/blood.V92.9.3318 [9] Nishimura R, Baker J, Beilhack A, et al. In vivo trafficking and survival of cytoki-ne-induced killer cells resulting in minimal GVHD with retention of anti-tumor activity[J]. Blood, 2008, 112(6): 2563-2574. doi: 10.1182/blood-2007-06-092817 [10] Edinger M, Cao YA, Verneris MR, et al. Revealing lymphoma growth and the efficacy of immune cell therapies using in vivo bioluminescence imaging[J]. Blood, 2003, 101(2): 640-648. doi: 10.1182/blood-2002-06-1751 [11] Thorne SH, Negrin RS, Contag CH. Synergistic antitumor effects of immune cell-viral biotherapy[J]. Science, 2006, 311(5768): 1780-1784. doi: 10.1126/science.1121411 [12] Marin V, Dander E, Biagi E, et al. Characterization of in vitro migratory properties of anti-CD19 chimeric receptor-redirected CIK cells for their potential use in B-ALL immunotherapy[J]. Exp Hematol, 2006, 34(9): 1219-1229. http://www.onacademic.com/detail/journal_1000035044546310_4fc0.html [13] Jiang JT, Shen YP, Wu CP, et al. Increasing the frequency of CIK cells adoptive immunotherapy may decrease risk of death in gastric cancer patients[J]. World J Gastroenterol, 2010, 16(48): 6155-6162. doi: 10.3748/wjg.v16.i48.6155 [14] Su X, Zhang L, Jin L, et al. Immunotherapy with cytokine-induced killer cells in metastatic renal cell carcinoma[J]. Cancer Biother Radiopharm, 2010, 25(4): 465-470. doi: 10.1089/cbr.2010.0762 [15] Takayama T, Sekine T, Makuuchi M, et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial[J]. Lancet, 2000, 356(9232): 802-807. doi: 10.1016/S0140-6736(00)02654-4 [16] 司徒杰, 高新, 湛海伦, 等. CIK细胞免疫疗法在肾癌治疗中的应用[J]. 白求恩军医学院学报, 2005, 3(4): 215-216. doi: 10.3969/j.issn.1672-2876.2005.04.013 [17] Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase Ⅲ trial[J]. Lancet, 2009, 373(9674): 1525-1531. doi: 10.1016/S0140-6736(09)60569-9 [18] Schmidt-Wolf IG, Lefterova P, Mehta BA, et al. Phenotypic characterization and identification of effector cells involved in tumor cell recognition of cytokine-ind-uced killer cells[J]. Exp Hematol, 1993, 21(13): 1673-1679. http://www.ncbi.nlm.nih.gov/pubmed/7694868/ -




 下载:
下载: