-
摘要:
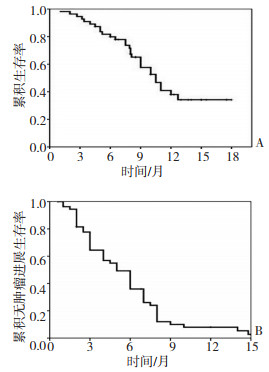
目的 评价索拉非尼治疗进展期肝细胞癌(HCC)的疗效及分析其预后影响因素。 方法 前瞻性分析2007年8月至2009年7月间110例接受索拉非治疗的进展期HCC患者,评价其疗效、不良反应,以总生存期和无肿瘤进展生存期为预后指标进行单因素和Cox比例风险模型多因素分析。 结果 110例患者随访中位时间9(2~18)个月,服用索拉非尼中位时间6.5(2~18)个月。14例(12.7%)获得完全缓解(CR),16例(14.5%)部分缓解(PR),40例(36.4%)病情稳定(SD),总有效率为70例(63.6%)。中位生存期和无肿瘤进展生存期分别为10.5个月(95%CI:8.7~12.3)和5.0个月(95%CI:3.7~6.3)。多因素分析显示:联合局部治疗(肝动脉化疗栓塞或氩氦刀)、美国东部肿瘤协作组活动状态评分(Eastern Cooperative Oncology Group performance status score,ECOG PS)和Child-Pugh分级是影响无肿瘤进展生存时间的独立预后因素,而联合局部治疗、ECOG PS评分和AFP(alfa-fetopro? tein)水平是影响总生存期的独立预后因素。亚组分析显示:在肝癌进展组患者中继续服用索拉非尼其总生存期明显长于终止索拉非尼治疗者(11个月vs. 7.5个月,P < 0.001)。 结论 索拉非尼治疗进展期HCC,ECOG PS评分是影响生存期的一个重要因素,联合局部治疗有益于改善生存期。 -
关键词:
- 肝细胞癌 /
- 索拉非尼 /
- 预后 /
- 经导管肝动脉化疗栓塞 /
- 氩氦刀
Abstract:Objective To evaluate the efficacy and analyze the prognostic factors of sorafenib treatment in patients with advanced hepatocellular carcinoma (HCC). Methods Baseline characteristics and outcomes of 110 patients with advanced HCC treated with sorafenib with/without local therapy from a single liver cancer center were collected. Predictors of progress-free survival (PFS) and overall survival (OS) were determined by multivariable analysis. Results Complete response was observed in 14 patients (12.7%), partial response (PR) in 16 patients (14.5%), and stable disease (SD) in 40 patients (36.4%). The therapeutic effective rate was 63.6%. The median OS and PFS for the entire cohort were 10.5 months (95% CI, 8.7-12.3 months) and 5.0 months (95% CI, 3.7-6.3 months), respectively. Sorafenib combined with local treatment (transarterial chemoembolization with/without cryoablation) was an independent predictor of good PFS. Two negative factors, namely, the Eastern Cooperative Oncology Group (ECOG) performance status (PS) and Child-Pugh class, predicted poor PFS independently. The ECOG PS and alfa-fetoprotein were found to be independent adverse predictors of the OS, whereas the combination of local treatment was an independent predictor of good OS. In a subset of patients with progressive disease, a significant difference was found between the OS rates of patients with continuous sorafenib treatment and those who discontinued the therapy (11 months vs. 7.5 months, P < 0.001). Conclusion The ECOG PS is an important predictor for survival rate of patients with advanced HCC. Sorafenib combined with local therapy may achieve better outcomes for these patients. The survival benefit of the combined treatment of sorafenib with local therapy warrants further studies. -
表 1 影响索拉非尼治疗进展期肝癌总生存期和无肿瘤进展生存期的单因素分析
Table 1. Univariate analysis of factors affecting OS and PFS of advanced HCC patients treated with sorafenib

表 2 进展期肝癌患者继续服用索拉非尼与停用索拉非尼两组临床资料分析
例 Table 2. Characteristics of HCC patients with radiologic PD between the group with continuation of sorafenib treatment and that with stopping sorafenib therapy

-
[1] Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma[J]. The Lancet, 2003, 362(9399): 1907-1917. doi: 10.1016/S0140-6736(03)14964-1 [2] Palmer DH, Hussain SA, Johnson PJ. Systemic therapies for hepatocellular carcinoma[J]. Expert Opin Invest Drugs, 2004, 13(12): 1555-1568. doi: 10.1517/13543784.13.12.1555 [3] Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma[J]. Semin Liver Dis, 2010, 30(1): 52-60. doi: 10.1055/s-0030-1247132 [4] 杨秉辉, 任正刚. 原发性肝癌诊断标准[J]. 中华肝脏病杂志, 2000, 8(3): 135-137. doi: 10.3760/j.issn:1007-3418.2000.03.002 [5] Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification[J]. Semin Liver Dis, 1999, 19(3): 329-338. doi: 10.1055/s-2007-1007122 [6] 王春平, 陆荫英, 王新真, 等. 经皮氩氦刀冷冻消融治疗原发性肝癌[J]. 解放军医学杂志, 2008, 33(12): 1399-1404. doi: 10.3321/j.issn:0577-7402.2008.12.001 [7] Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment[J]. Semin Radiat Oncol, 2003, 13(3): 176-181. doi: 10.1016/S1053-4296(03)00031-6 [8] Abou-Alfa GK, Letourneau R, Harker G, et al. Randomized phase Ⅲ study of exatecan and gemcitabine compared with gemcitabine alone in untreated advanced pancreatic cancer[J]. J Clin Oncol, 2006, 24(27): 4441-4447 doi: 10.1200/JCO.2006.07.0201 [9] Yau T, Chan P, Ng KK, et al. Phase 2 open-label study of single-agent sorafenib in treating advanced hepatocellular carcinoma in a hepatitis B-endemic Asian population: presence of lung metastasis predicts poor response[J]. Cancer, 2009, 115(2): 428-436. doi: 10.1002/cncr.24029 [10] Rabe C, Pilz T, Klostermann C, et al. Clinical characteristics and outcome of a cohort of 101 patients with hepatocellular carcinoma [J]. World J Gastroenterol, 2001, 7(2): 208-215. doi: 10.3748/wjg.v7.i2.208 [11] Han KH, Seong J, Kim JK, et al. Pilot clinical trial of localized concurrent chemoradiation therapy for locally advanced hepatocellular carcinoma with portal vein thrombosis[J]. Cancer, 2008, 113(5): 995-1003. doi: 10.1002/cncr.23684 [12] Cheng AL, Kang YK, Chen Z, et al. Randomized Phase Ⅲ Trial of Sorafenib Versus Placebo in Asian Patients With advanced Hepatocellular Carcinoma[J]. Lancet Oncol, 2009, 10(1): 25-34. doi: 10.1016/S1470-2045(08)70285-7 [13] Dufour JF, Hoppe H, Heim MH, et al. Continuous administration of sorafenib in combination with transarterial chemoembolization in patients with hepatocellular carcinoma: results of a phase I study [J]. Oncologist, 2010, 15(11): 1198-1204. doi: 10.1634/theoncologist.2010-0180 [14] Furuse J, Ishii H, Nakachi K, et al. Phase I study of sorafenib in Japanese patients with hepatocellular carcinoma[J]. Cancer Sci, 2008, 99 (1): 159-165. [15] Hakimé A, Hines-Peralta A, Peddi H, et al. Combination of radiofrequency ablation with antiangiogenic therapy for tumor ablation efficacy: study in mice[J]. Radiology, 2007, 244(2): 464-470. doi: 10.1148/radiol.2442061005 [16] Poon RT, Ng IO, Lau C, et al. Correlation of serum basic fibroblast growth factor levels with clinicopathologic features and postoperative recurrence in hepatocellular carcinoma[J]. Am J Surg, 2001, 182 (3): 298-304. doi: 10.1016/S0002-9610(01)00708-5 [17] Ng IO, Poon RT, Lee JM, et al. Microvessel density, vascular endothelial growth factor and its receptors Flt-1 and Flk-1/KDR in hepatocellular carcinoma[J]. Am J Clin Pathol, 2001, 116(6): 838-845. doi: 10.1309/FXNL-QTN1-94FH-AB3A [18] Wörns MA, Weinmann A, Pfingst K, et al. Safety and efficacy of sorafenib in patients with advanced hepatocellular carcinoma in consideration of concomitant stage of liver cirrhosis[J]. J Clin Gastroentero, 2009, 43(5): 489-495. doi: 10.1097/MCG.0b013e31818ddfc6 [19] Grothey A, Hedrick EE, Mass RD, et al. Response-independent survival benefit in metastatic colorectal cancer: a comparative analysis of N9741 and AVF2107[J]. J ClinOncol, 2008, 26(2): 183-189. doi: 10.1200/JCO.2007.13.8099 [20] Ozenne V, Paradis V, Pernot S, et al. Tolerance and outcome of patients with unresectable hepatocellular carcinoma treated with sorafenib[J]. Eur J Gastroenterol Hepatol, 2010, 22(9): 1106-1110. doi: 10.1097/MEG.0b013e3283386053 [21] Morimoto M, Numata K, Kondo M, et al. Higher discontinuation and lower survival rates are likely in elderly Japanese patients with advanced hepatocellular carcinoma receiving sorafenib[J]. Hepatol Res, 2011, 41(4): 296-302. doi: 10.1111/j.1872-034X.2011.00778.x [22] Li M, Li H, Li C, et al. Cytoplasmic alpha-fetoprotein functions as a co-repressor in RA-RAR signaling to promote the growth of human hepatoma Bel 7402 cells[J]. Cancer Lett, 2009, 285(2): 190-199. doi: 10.1016/j.canlet.2009.05.014 [23] Li M, Li H, Li C, et al. Alpha fetoprotein is a novel protein-binding partner for caspase-3 and blocks the apoptotic signaling pathway in human hepatoma cells[J]. Int J Cancer, 2009, 124(12): 2845-2854. doi: 10.1002/ijc.24272 -




 下载:
下载: