Gemcitabine/Docetaxel Combination as Second-line Treatment for 11 Advanced Soft Tissue Sarcoma Patients
-
摘要:
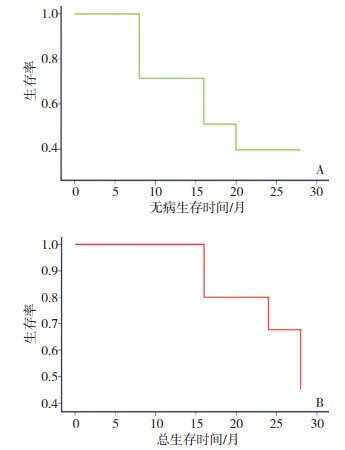
目的 探讨吉西他滨联合多西他赛治疗晚期软组织肉瘤的临床疗效。 方法 收集11例对一线化疗药物耐药的晚期软组织肉瘤患者,应用吉西他滨(剂量为900 mg/m2,第1和第8天)联合多西他赛(剂量为100 mg/m2,第8天)每3周重复给药。如患者曾经接受放疗,吉西他滨剂量减为675 mg/m2,多西他赛剂量减为75 mg/m2。 结果 接受化疗的患者总有效率(CR+PR+SD)为54.5%。中位随访时间为19(1~36)个月,中位总生存期为15.4个月(95%CI:8.012~21.598)和中位无进展生存期为8.4个月(95%CI:7.342~8.768)。12个月和36个月的生存率分别是81.8%和27.3%。吉西他滨联合多西他赛治疗软组织肉瘤最常见的非血液学不良反应是黏膜炎,对症处理明显好转。 结论 吉西他滨联合多西他赛作为二线化疗药物治疗晚期软组织肉瘤患者具有较好的临床疗效,且有较好的耐受性,本研究结论为该方案的大样本临床试验提供了依据。 Abstract:Objective To investigate the promising anti-tumor effects of gemcitabine and/or docetaxel on metastatic or unresectable soft tissue sarcomas. Methods Data of 11 patients with advanced soft tissue sarcomas refractory to first-line chemotherapy treatment were enrolled in this study. They received gemcitabine alone or gemcitabine/docetaxel combination as follows: 900 mg/m2 gemcitabine on days 1 and 8, and 100 mg/m2 docetaxel on day 8. The regimen was repeated every 3 weeks. When irradiation was conducted before drug therapy, the doses were reduced to 675 mg/m2 gemcitabine on days 1 and 8, and 75 mg/m2 docetaxel on day 8. The regimen was repeated every 3 weeks. Results The gemcitabine/docetaxel combination was well tolerated. The most commonly observed hematologic toxicity of the combined therapy was neutropenia (45.5 %). The most common non-hematologic toxicity observed was mucositis (45.5 %). The overall response was 45.5%. Follow-ups ranging from 1 month to 36 months (median = 19 months) revealed that the median overall survival time was 15.4 months (95% CI = 8.012 - 21.598) and the median progression-free survival time was 8.4 months (95 % CI = 7.342 - 8.768). The one- and two-year survival rates were 81.8 % and 27.3 %, respectively. Conclusion Conclusion: A gemcitabine/docetaxel regimen as second-line treatment for patients with advanced soft tissues sarcomas is effective and has acceptable toxicities. These results should be evaluated in a large-sample Ⅲ trial. -
Key words:
- Advanced soft tissue sarcomas /
- Second-line chemotherapy /
- Gemcitabine /
- Docetaxel
-
表 1 11例接受吉西他滨联合多西他赛化疗患者的临床资料
Table 1. Clinical data of 11 patients subjected to gemcitabine/docetaxel combination chemotherapy

表 2 吉西他滨联合多西他赛化疗引起的不良反应 例(%)
Table 2. The hematologic toxicity of gemcitabine/docetaxel combination

-
[1] Singer S, Demetri GD, Baldini EH, et al. Management of soft-tissue sarcomas: an overview and update[J]. Lancet Oncol, 2000, 1: 75-85. doi: 10.1016/S1470-2045(00)00016-4 [2] Clark MA, Fisher C, Judson I, et al. Soft-tissue sarcomas in adults [J]. N Engl J Med, 2005, 353(7): 701-711. doi: 10.1056/NEJMra041866 [3] Sleijfer S, Ouali M, van Glabbeke M, et al. Prognostic and predictive factors for outcome to first-line ifosfamide-containing chemotherapy for adult patients with advanced soft tissue sarcomas: an exploratory, retrospective analysis on large series from the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STBSG)[J]. Eur J Cancer, 2010, 46(1): 72-83. doi: 10.1016/j.ejca.2009.09.022 [4] Fowler JD, Brown JA, Johnson KA, et al. Kinetic investigation of the inhibitory effect of gemcitabine on DNA polymerization catalyzed by human mitochondrial DNA polymerase[J]. J Biol Chem, 2008, 283(22): 15339-15348. doi: 10.1074/jbc.M800310200 [5] Look KY, Sandler A, Blessing JA, et al. Phase Ⅱ trial of gemcitabine as second-line chemotherapy for uterine leiomyosarcoma: a Gynecologic Oncology Group (GOG) Study[J]. Gynecol Oncol, 2004, 92 (2): 644-647. doi: 10.1016/j.ygyno.2003.11.023 [6] Lee HY, Shin SJ, Kim HS, et al. Weekly gemcitabine and docetaxel in refractory soft tissue sarcoma: a retrospective analysis[J]. Cancer Res Treat, 2012, 44(1): 43-49. doi: 10.4143/crt.2012.44.1.43 [7] Hensley ML, Maki R, Venkatraman E, et al. Gemcitabine and docetaxel in patients with unresectable leiomyosarcoma: results of a phase Ⅱ trial[J]. J Clin Oncol, 2002, 20(12): 2824-2831. doi: 10.1200/JCO.2002.11.050 [8] Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment[J]. Semin Radiat Oncol, 2003, 13(3): 176-181. doi: 10.1016/S1053-4296(03)00031-6 [9] Italiano A, Toulmonde M, Lortal B, et al. "Metronomic" chemotherapy in advanced soft tissue sarcomas[J]. Cancer Chemother Pharmacol, 2010, 66(1): 197-202. doi: 10.1007/s00280-010-1275-3 [10] Maurel J, López-Pousa A, de Las Peñas R, et al. Efficacy of sequential high-dose doxorubicin and ifosfamide compared with standard-dose doxorubicin in patients with advanced soft tissue sarcoma: an open-label randomized phase Ⅱ study of the Spanish group for research on sarcomas[J]. J Clin Oncol, 2009, 27(11): 1893-1898. doi: 10.1200/JCO.2008.19.2930 [11] Leu KM, Ostruszka LJ, Shewach D, et al. Laboratory and clinical evidence of synergistic cytotoxicity of sequential treatment with gemcitabine followed by docetaxel in the treatment of sarcoma[J]. J Clin Oncol, 2004, 22(9): 1706-1712. doi: 10.1200/JCO.2004.08.043 [12] Bay JO, Ray-Coquard I, Fayette J, et al. Docetaxel and gemcitabine combination in 133 advanced soft-tissue sarcomas: a retrospective analysis[J]. Int J Cancer, 2006, 119(3): 706-711. doi: 10.1002/ijc.21867 [13] Maki RG, Wathen JK, Patel SR, et al. Randomized phase Ⅱ study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002[J]. J Clin Oncol, 2007, 25(19): 2755-2763. doi: 10.1200/JCO.2006.10.4117 -




 下载:
下载: