Regulatory effect of 17-AAG on VEGF expression in gastric cancer cells via STAT3 signaling pathway
-
摘要:
目的 观察17-烯丙胺-17-脱甲氧格尔德霉素(17-allylamino-17-desmethoxy-geldanamycin, 17-AAG)对人胃癌MKN-45细胞信号转导和转录活化蛋白3(signal transducer and activator of transcriptions 3, STAT3)、血管内皮生长因子(vascularendothelial growth factor, VEGF)mRNA及蛋白表达水平的影响, 并探寻STAT3通路所发挥的作用。 方法 体外培养人胃癌MKN-45细胞, 分别给予不同剂量及不同作用时间17-AAG进行作用, 四甲基偶氮唑盐微量酶反应比色法(MTT法)检测细胞增殖能力; RT-PCR法和Western Blotting法检测各组细胞STAT3和VEGF mRNA和蛋白的表达水平。 结果 0.165~10 mg/L的17-AAG作用24、48 h后对MKN-45细胞有显著的抑制作用, 且有明显的时间、剂量依赖性; 1.0、2.0、3.0、5.0 mg/L的17-AAG作用48 h后, 各组细胞STAT3和VEGF mRNA和蛋白表达均下调, 且具有浓度依赖性; 3.0 mg/L 17-AAG作用12、24、48h后, 各组细胞STAT3和VEGF mRNA和蛋白表达均下调, 且具有时间依赖性。 结论 17-AAG对人胃癌MKN-45细胞的增殖具有明显的抑制作用, 并能抑制STAT3和VEGF mRNA和蛋白的表达, 17-AAG可能通过对STAT3通路的负性调控作用而抑制VEGF表达。 Abstract:Objective This study aims to investigate the effects of 17-allylamino-17-desmethoxy-geldanamycin(17-AAG) on the gene and protein expressions of VEGF and STAT3 pathways in MKN-45 human gastric cancer cells, and explore the potential mechanisms of the STAT3 signaling pathway that mediate these effects. Methods MKN-45 cells were seeded in Dulbecco's Modified Eagle Medium.The thiazolyl blue method, i.e., methyl thiazolyl tetrazolium assay, was performed to evaluate the inhibitory effects of 17-AAG on the proliferation of MKN-45 cells at different times, with different doses.The gene and protein expression of VEGF and STAT3 were determined by reverse transcription polymerase chain reaction(RT–PCR) and Western blot analysis. Results MKN-45 cells were treated with 17-AAG at 0.165 mg/L to 10 mg/L for 24 and 48 h, respectively.17-AAG significantly inhibited MKN-45 cell proliferation in a time-and dose-dependent manner.The results of the RT–PCR and Western blot assays showed that the gene and protein expressions of VEGF and STAT3 in MKN-45 cells induced by 17-AAG were significantly down-regulated in a dose-dependent manner when the cells were treated with 17-AAG at 1.0, 2.0, 3.0, and 5.0 mg/L for 48 h.The gene and protein expressions of VEGF and STAT3 in the MKN-45 cells were significantly down-regulated in a time-dependent manner when the cells were treated with 17-AAG at 3.0 mg/L for 12, 24, and 48 h. Conclusion The drug 17-AAG can inhibit cell proliferation and down-regulate the gene and protein expressions of VEGF and STAT3 in MKN-45 human gastric cancer cells in vitro; the down-regulation of VEGF expression may be associated with the STAT3 signaling pathway. -
Key words:
- 17-AAG /
- cell proliferation /
- VEGF /
- STAT3
-
表 1 RT-PCR引物序列和产物大小及反应条件
Table 1. Primer sequences, products size and conditions for RT-PCR

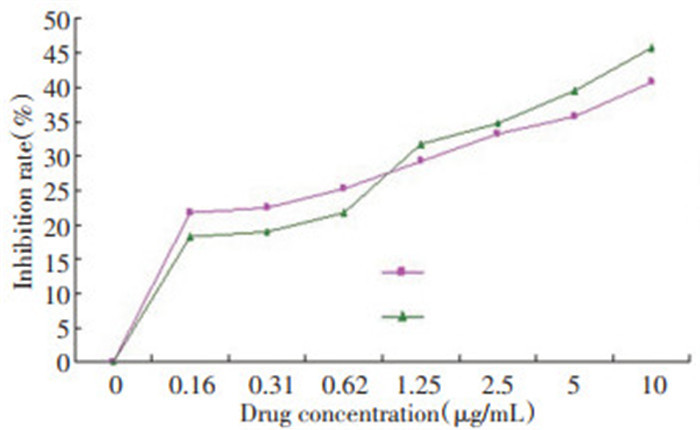
表 2 17-AAG体外不同作用时间对MKN-45细胞抑制率(n=5,x±s,%)
Table 2. Inhibition ratio of 17-AAG at various action times in vitro on MKN-45 cells

表 3 不同作用浓度17- AAG对MKN-45细胞系VEGF、STAT3基因mRNA表达的影响(x±s)
Table 3. Effect of various concentrations of 17-AAG on STAT3 and VEGF gene mRAN expressions in MKN-45 cells

表 4 不同时间17-AAG对人MKN-45细胞STAT3、VEGF mRNA表达的影响(x±s,n=5)
Table 4. Effect of various concentrations of 17-AAG on STAT3 and VEGF protein expressions in MKN-45 human cells

表 5 不同浓度17-AAG对人MKN-45细胞系STAT3、VEGF蛋白表达的影响(x±s,n=5)
Table 5. Effect of 17-AAG at various action times on STAT3 and VEGF mRNA expressions in MKN-45 human cells

表 6 不同时间17-AAG对人MKN-45细胞系STAT3、VEGF蛋白表达的影响(x±s,n=5)
Table 6. Effect of 17-AAG at various action times on STAT3 and VEGF protein expressions in MKN-45 human cells

-
[1] Zheng HC, Xu XY, Yu M, et al. The role of Reg IV gene and its encoding product in gastric carcinogenesis[J]. Hum Pathol, 2010, 41: 59-69. doi: 10.1016/j.humpath.2009.06.013 [2] Page BD, Ball DP, Gunning PT. Signal transducer and activator of transcription 3 inhibitors: a patent review[J]. Expert Opin Ther Pat, 2011, 21(1): 65-83. doi: 10.1517/13543776.2011.539205 [3] Spano JP, Milano G, Rixe C, et al. JAK/STAT signalling pathwayin colorectal cancer: a new biological targetwith therapeutic implica tions[J]. Eur J Cancer, 2006, 42(16): 2668-2670. doi: 10.1016/j.ejca.2006.07.006 [4] Herrmann A, Kortylewski M, Kujawski M, et al. Targeting Stat3 in themyeloid com partment drasticallyim proves the invivo antitumor functions of adoptively transferred T cells[J]. Cancer Res, 2010, 70(19): 7455-7464. doi: 10.1158/0008-5472.CAN-10-0736 [5] Rivat C, Rodrigues S, Bruyneel E, et al. Implication of STAT3 sig naling in human colonic cancer cells during intestinal trefoil factor 3 (TFF3)-and vascular endothelial growth factor-mediated cellular invasion and tumor growth[J]. Cancer Res, 2005, 65(1): 195-202. [6] Okazaki H, Tokumaru S, Hanakawa Y, et al. Nuclear translocation of phosphorylated STAT3 regulates VEGF-A-induced lymphatic endothelial cell migration and tube formation[J]. Biochem Biophys Res Commun, 2011, 412(3): 441-445. doi: 10.1016/j.bbrc.2011.07.111 [7] Zheng Z, Chen H, Zhao H, et al. JAK2/STAT3 signaling pathway activation mediates tumor angiogenesis by upregulation of VEGF and bFGF in non-small-cell lung cancer[J]. Invest Ophthalmol Vis Sci, 2010, 51(1): 64-71. doi: 10.1167/iovs.09-3511 [8] Goydos JS. Vascular endothelial growth factor C mRNA expres sion correlates with stage of progression in patients with melanoma[J]. Clin Cancer Res, 2003, 9(16): 5962-5967. [9] Modi S, Stopeck A, Linden H, et al. HSP90 inhibition is effective inbreast cancer: a phase II trial of tanespimycin(17-AAG) plus trastu zumab in patients with HER2-positive metastatic breast cancer pro gressing on trastuzumab[J]. Clin Cancer Res, 2011, 17(15): 5132-5139. doi: 10.1158/1078-0432.CCR-11-0072 [10] Pacey S, Gore M, Chao D, et al. A Phase II trial of 17-allylamino, 17-demethoxygeldanamycin (17-AAG, tanespimycin) in patients with metastatic melanoma[J]. Invest New Drugs, 2012, 30(1): 341-349. doi: 10.1007/s10637-010-9493-4 [11] Cheong JH, Hong SY, Zheng Y, et al. Eupatilin Inhibits Gastric Cancer Cell Growth by Blocking STAT3-Mediated VEGF Expres sion[J]. J Gastric Cancer, 2011, 11(1): 16-22. doi: 10.5230/jgc.2011.11.1.16 -




 下载:
下载: