Effect of silencing HIF-2α expression on the enrichment of breast cancer stem cell microspheres under hypoxia
-
摘要:
目的 探讨沉默低氧诱导因子-2α(hypoxia-inducible factor-2α, HIF-2α)表达对低氧下乳腺癌干细胞(breast cancerstem cells, BCSCs)微球体富集的影响。 方法 构建HIF-2αRNA干扰慢病毒载体, 并转染至MCF-7细胞, RT-PCR和Western blot检测HIF-2αmRNA及蛋白表达; MTT检测MCF-7细胞在不同氧浓度下增殖活性; 显微镜下观察低氧下BCSCs微球体形成情况; 有限稀释法检测微球体细胞单克隆形成能力; RT-PCR检测微球体细胞干细胞相关标志物ABCG2、CD44及OCT-4 mRNA表达。 结果 成功构建了HIF-2α基因siRNA慢病毒表达载体, RT-PCR和Western blot结果显示干扰组细胞HIF-2αmRNA及蛋白表达均明显降低(P < 0.05)。与未转染组及空载体组比较, 低氧下RNA干扰组细胞增殖活性及BCSCs单克隆形成能力明显降低(P < 0.05);RT-PCR结果亦显示RNA干扰组BCSCs相关标志物ABCG2、CD44及OCT-4 mRNA表达显著降低(P < 0.05)。 结论 低氧能够诱导和强化MCF-7细胞乳腺癌癌干细胞样特性, 而沉默HIF-2α表达可抑制低氧下BCSCs富集, 提示HIF-2α有望成为乳腺癌治疗新的靶点。 Abstract:Objective This work aims to explore the effect of silencing hypoxia-inducible factor(HIF)-2α expression on the enrichment of breast cancer stem cell(BCSC) microspheres under hypoxia. Methods A lentiviral vector of the RNA interference(RNAi) of human HIF-2 α gene was constructed and transfected into Michigan Cancer Foundation(MCF)-7 cells.The mRNAand protein expressions of HIF-2α were determined using real time-polymerase chain reaction(RT-PCR) and Western blot, respectively.The proliferation activity of the MCF-7 cells under different oxygen concentrations was measured using the methyl thiazolyl tetrazolium assay.The formation of the BCSC microspheres under hypoxia was observed by a fluorescence microscope.The monoclonal formation ability of the microsphere cells was detected by limiting dilution assay.The mRNA expressions of the BCSC markers, ABCG, CD44, and OCT-4, were tested by RT-PCR. Results The siRNA expression vector targeting the HIF-2α gene was successfully constructed.Both the HIF-2a mRNA and protein levels noticeably decreased in the MCF-7 cells transfected with the RNAi expression vector(P < 0.05).Compared with the groups with empty vectors or those that were not transfected, the proliferation activity of MCF-7 cells and the monoclonal formation ability of BCSCs in the RNAi group were markedly inhibited after silencing the HIF-2α gene(P < 0.05).RT-PCR results showed that the mRNA expressions of BCSC markers ABCG2, CD44, and OCT-4 in cells from the RNAi group decreased significantly under hypoxia(P < 0.05). Conclusion Hypoxia induced and enhanced cancer stem cell-like characteristics in MCF-7 cells.HIF-2α silencing leads to the decreased enrichment of BCSC microspheres under hypoxia.HIF-2 α may be a new candidate gene for BCSC-targeting therapy. -
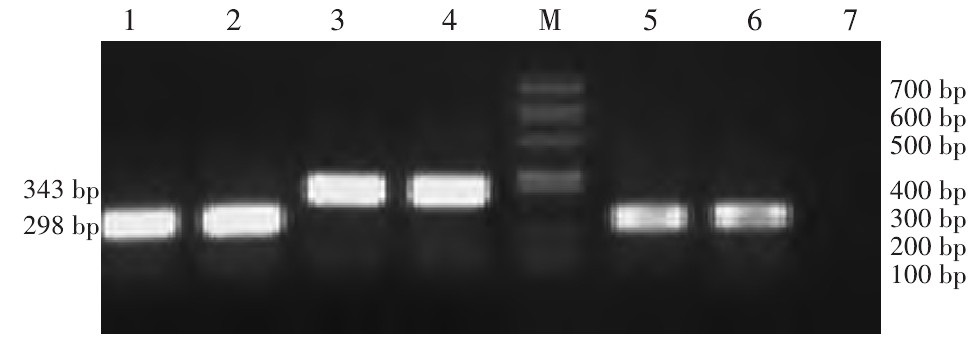
图 2 RT-PCR和Western blot检测各组MCF-7细胞中HIF-2 α mRNA及蛋白表达
Figure 2. HIF-2α mRNA and protein expressions in MCF-7 cells from different groups, as detected by RT-PCR and Western blot, respectively (x±s, n=3)
1:RNA interference group(20% O2); 2:RNA interference group(1% O2)3:Empty vector group(20% O2); 4:Empty vector group(1% O2); M: Marker
图 4 荧光显微镜下观察低氧下各组MCF-7细胞微球体形成情况
Figure 4. Microsphere formation of MCF-7 cells for different groups un-der hypoxia, as observed by fluorescent microscopy
A: Non-transfected group(×40);B: Empty vector group(×40);C: RNA interference group(×40);D: Non-transfected group(×200);E: Empty vector group(×200);F: RNA interference group(×200)
-
[1] Nurwidya F, Takahashi F, Minakata K, et al. From tumor hypoxiato cancer progression: the implications of hypoxia-inducible factor-1 expression in cancers[J]. Anat Cell Biol, 2012, 45(2): 73-78. doi: 10.5115/acb.2012.45.2.73 [2] 王秀超, 苑占娜, 李莎莎, 等. 低氧诱导因子-2α在肝细胞癌中的表达及其临床意义[J]. 中国肿瘤临床, 2011, 38(10): 560-563. doi: 10.3969/j.issn.1000-8179.2011.10.006 [3] Loboda A, Jozkowicz A, Dulak J. HIF-1 and HIF-2 transcriptionfactors-similar but not identical[J]. Mol Cells, 2010, 29(5): 435-442. doi: 10.1007/s10059-010-0067-2 [4] Noman MZ, Messai Y, CarréT, et al. Microenvironmental hypoxia orchestrating the cell stroma cross talk, tumor progression and antitumor response[J]. Crit Rev Immunol, 2011, 31(5): 357-377. doi: 10.1615/CritRevImmunol.v31.i5.10 [5] Zhu P, Ning Y, Yao L, et al. The proliferation, apoptosis, invasionof endothelial-like epithelial ovarian cancer cells induced by hypoxia[J]. J Exp Clin Cancer Res, 2010, 29: 124. doi: 10.1186/1756-9966-29-124 [6] 罗维远, 黄正接, 罗琪. 乳腺癌干细胞研究的新进展[J]. 中国肿瘤临床, 2011, 38(23): 1475-1478. doi: 10.3969/j.issn.1000-8179.2011.23.015 [7] Persano L, Rampazzo E, Basso G, et al. Glioblastoma cancer stemcells: Role of the microenvironment and therapeutic targeting[J]. Biochem Pharmacol, 2012[Epub ahead of print]. [8] Zeng W, Wan R, Zheng Y, et al. Hypoxia, stem cells and bone tumor[J]. Cancer Lett, 2011, 313(2): 129-136. doi: 10.1016/j.canlet.2011.09.023 [9] Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors andthe response to hypoxic stress[J]. Mol Cell, 2010, 40(2): 294-309. doi: 10.1016/j.molcel.2010.09.022 [10] Keith B, Johnson RS, Simon MC. HIF1αand HIF2α: sibling rivalryin hypoxic tumor growth and progression[J]. Nat Rev Cancer, 2011, 12(1): 9-22. [11] Li M, Kim WY. Two sides to every story: the HIF-dependent andHIF-independent functions of pVHL[J]. J Cell Mol Med, 2011, 15(2): 187-195. doi: 10.1111/j.1582-4934.2010.01238.x [12] Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy[J]. Trends Pharmacol Sci, 2012, 33(4): 207-214. doi: 10.1016/j.tips.2012.01.005 [13] Li Z, Bao S, Wu Q, et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem Cells[J]. Cancer Cell, 2009, 15(6): 501-513. doi: 10.1016/j.ccr.2009.03.018 [14] Pietras A, Hansford LM, Johnsson AS, et al. HIF-2alpha maintainsan undifferentiated state in neural crest-like human neuroblastomatumor-initiating cells[J]. Proc Natl Acad Sci U S A, 2009, 106(39): 16805-16810. doi: 10.1073/pnas.0904606106 [15] Hochedlinger K, Yamada Y, Beard C, et al. Ectopic expression ofOct-4 blocks progenitor-cell differentiation and causes dysplasiain epithelial tissues[J]. Cell, 2005, 121(3): 465-477. doi: 10.1016/j.cell.2005.02.018 -




 下载:
下载: