Regulation of CX3CR1 expression in pancreatic cancer cells by hypoxia inducible factor 1α
-
摘要:
目的 以胰腺癌细胞株Patu8988为研究对象, 通过过表达和干扰低氧诱导因子(HIF-1α), 观察CX3CR1表达水平的变化, 并探讨CX3CR1在胰腺癌中的调控机制。 方法 分别构建pcDNA3.1-HIF-1α过表达质粒和HIF-1α-siRNA, 转染胰腺癌细胞株Patu8988, 经Western blot、半定量PCR检测CX3CR1的表达情况。采用染色质免疫沉淀(chromatin immunoprecipitation, ChIP)、荧光素酶技术探查HIF-1α与CX3CR1启动子区的结合情况。 结果 Patu8988转染pcDNA3.1-HIF-1α后CX3CR1的表达增加, 敲除HIF-1α后CX3CR1的表达减少。HIF-1α与CX3CR1启动子区的低氧反应元件直接结合, 并上调CX3CR1启动子的活性(P < 0.01)。 结论 HIF-1α调控CX3CR1在胰腺癌细胞中的表达。 Abstract:Objective This study aimed to investigate the effect of hypoxia inducible factor 1α(HIF-1α) expression on CX3CR1 and its regulatory mechanism in pancreatic cancer cell line Patu8988. Methods The highly expressed plasmid pcDNA3.1 HIF-1α and siRNA HIF-1α were initially constructed. After the plasmid was separately transfected to the pancreatic cancer cells, CX3CR1 and HIF-1α expressions were assayed by western blot analysis and real-time quantitative reverse transcriptase-polymerase chain reaction. The relationship between HIF-1α and CX3CR1 promoter was determined by chromatin immunoprecipitation and luciferase technology. Results The overexpressed HIF-1α could upregulate the CX3CR1 expression in pancreatic cancer cells. The CX3CR1 expression was significantly reduced when HIF-1 α was knocked down. Chromatin immunoprecipitation assay demonstrated that HIF-1α could be directly bound to the hypoxia-response element(5'-A/GCGTG-3') of the CX3CR1 promoter. This binding activity was significantly enhanced under hypoxic condition. CX3CR1 promoter-induced HIF-1α overexpression could significantly upregulate the expression of luciferase reporter genes in pancreatic cancer cells(P < 0.01). Conclusion HIF-1α could regulate CX3CR1 expression in pancreatic cancer cells. -
Key words:
- hypoxia inducible factor 1α /
- CX3CR1 /
- pancreatic cancer
-
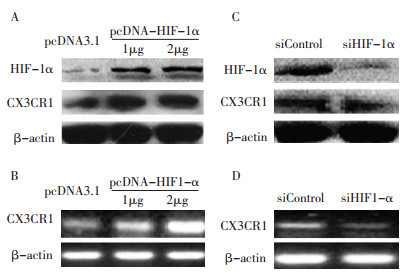
图 1 过表达和干扰HIF-1α前后CX3CR1的表达变化
Figure 1. Changes in CX3CR1 before and after pcDNA3.1 HIF-1α or siHIF-1α was overexpressed and transfected
A: Patu8988 cells transfected with pcDNA3.1 HIF-1α plasmids (1 μg, 2 μg) and assessed by Western blot analysis after 48 h; B: Patu8988 cells transfected with pcDNA3.1 HIF-1 α plasmids (1 μ g, 2 μ g) and assessed by semi-quantitative PCR after 48 h; C: Patu8988 cells transfected with siHIF-1 α (50 nM) and assessed by Western blot analysis after 48 h; D: Patu8988 cells transfected with siHIF-1α (50 nM) and assessed by semi-quantitative PCR after 48 h
图 2 HIF-1α直接结合CX3CR1启动子的HRE区并上调其活性
Figure 2. HIF-1αcould directly interact and upregulate CX3CR1 promoter activity
▶A: DNA sequence of the CX3CR1 promoter. Hypoxia-response elements were located at different sites; B: Chromatin immunoprecipitation analysis of Patu8988 cells. PCR products of the vascular endothelial growth factor promoter were used as the positive control sample. H, N, and NC indicate hypoxia, normoxia, and negative control, respectively; C: Luciferase analysis of Patu8988 cells. The cells were cotransfected with pGL3-CX3CR1 (1μg) and pcDNA3.1 HIF-1 α plasmids (1μg). Luciferase analysis was performed with a dual-luciferase reporter assay system. Y axis: pGL3-CX3CR1-related luciferase activity. **P < 0.01 versus the control group
-
[1] Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012[J]. CA Cancer J Clin, 2012, 62(1): 10-29. doi: 10.3322/caac.20138 [2] Gao YJ, Ji RR. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain[J]. Pharmacol Ther, 2010, 126 (1): 56-68. doi: 10.1016/j.pharmthera.2010.01.002 [3] Zhao T, Gao S, Wang X, et al. Hypoxia-inducible factor-1α regulates chemotactic migration of pancreatic ductal adenocarcinoma cells through directly transactivating the CX3CR1 gene[J]. PLoS One, 2012, 7(8): e43399. doi: 10.1371/journal.pone.0043399 [4] Marchesi F, Piemonti L, Fedele G, et al. The chemokine receptor CX3CR1 is involved in the neural tropism and malignant behavior of pancreatic ductal adenocarcinoma[J]. Cancer Res, 2008, 68(21): 9060-9069. doi: 10.1158/0008-5472.CAN-08-1810 [5] Imai T, Hieshima K, Haskell C, et al. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion[J]. Cell, 1997, 91(4): 521-530. doi: 10.1016/S0092-8674(00)80438-9 [6] Liebig C, Ayala G, Wilks JA, et al. Perineural invasion in cancer: a review of the literature[J]. Cancer, 2009, 115(15): 3379-3391. doi: 10.1002/cncr.24396 [7] Jamieson-Gladney WL, Zhang Y, Fong AM, et al. The chemokine receptor CX?CR1 is directly involved in the arrest of breast cancer cells to the skeleton[J]. Breast Cancer Res, 2011, 13(5): R91. doi: 10.1186/bcr3016 [8] Bazan JF, Bacon KB, Hardiman G, et al. A new class of membrane-bound chemokine with a CX3C motif[J]. Nature, 1997, 385 (6617): 640-644. doi: 10.1038/385640a0 [9] Pan Y, Lloyd C, Zhou H, et al. Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation[J]. Nature, 1997, 387(6633): 611-617. doi: 10.1038/42491 [10] Haskell CA, Cleary MD, Charo IF. Unique role of the chemokine domain of fractalkine in cell capture. Kinetics of receptor dissociation correlate with cell adhesion[J]. J Biol Chem, 2000, 275(44): 34183-34189. doi: 10.1074/jbc.M005731200 [11] Marchesi F, Locatelli M, Solinas G, et al. Role of CX3CR1/ CX3CL1 axis in primary and secondary involvement of the nervous system by cancer[J]. J Neuroimmunol, 2010, 224(1-2): 39-44. doi: 10.1016/j.jneuroim.2010.05.007 [12] Cardona AE, Pioro EP, Sasse ME, et al. Control of microglial neurotoxicity by the fractalkine receptor[J]. Nat Neurosci, 2006, 9(7): 917-924. doi: 10.1038/nn1715 [13] Marchesi F, Locatelli M, Solinas G, et al. Role of CX3CR1/ CX3CL1 axis in primary and secondary involvement of the nervous system by cancer[J]. J Neuroimmunol, 2010, 224(1-2): 39-44. doi: 10.1016/j.jneuroim.2010.05.007 [14] Mimura I, Tanaka T, Wada Y, et al. Pathophysiological response to hypoxia - from the molecular mechanisms of malady to drug discovery: epigenetic regulation of the hypoxic response via hypoxia-inducible factor and histone modifying enzymes[J]. J Pharmacol Sci, 2011, 115(4): 453-458. doi: 10.1254/jphs.10R19FM [15] Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics[J]. Oncogene, 2010, 29(5): 625-634. doi: 10.1038/onc.2009.441 -




 下载:
下载: