The clinical trial of induction chemotherapy with cisplatin and docetaxel followed by radiation concurrent with weekly cisplatin for locally advanced esophageal cancer
-
摘要:
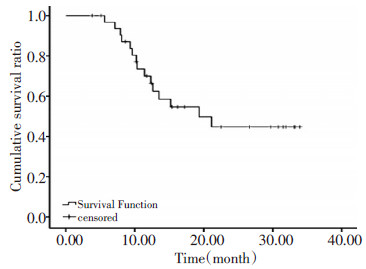
目的 研究不适手术的局部晚期食管癌患者行TP方案诱导化疗联合DDP同期放化疗的毒性及疗效。 方法 33例胸段食管鳞癌T3N0MO~T4N2M0期患者(不包括腹腔淋巴结转移),第1天和第22天行TP方案诱导化疗,多西他赛(艾素)75 mg/m2, DDP 75 mg/m2。第43天开始放疗,采用三维适形放疗,总剂量60 Gy,2 Gy/次,5次/周。同期化疗:DDP 30 mg/m2,1次/周,放疗开始的第1、8、15、22、29、36天给药。 结果 诱导化疗Ⅳ级骨髓毒性为12.12%(4/33),无Ⅲ级或以上的肝、肾毒性。同期放化疗骨髓毒性最高为Ⅲ级,红细胞、粒细胞、血小板Ⅲ级毒性分别为21.21%(7/33)、15.15%(5/33)、3.03%(1/33),无Ⅱ级以上的肝肾毒性。Ⅲ级放射性食管炎为9.10%(3/33),未发现Ⅲ级以上的放射性食管炎及Ⅰ级以上的急性放射性肺炎。治疗结束评价显效(CR+PR)84.85%(28/33),稳定(SD)12.12%(4/33),进展(PD)3.03%(1/33);治疗后2个月评价显效(CR+PR)75.76%(25/33),稳定(SD)9.10%(3/33),进展(PD)15.15%(5/33)。全组死亡病例15例。1年生存率66.4%,最主要失败模式是局部失败46.67%(7/15),局部+远处失败26.67%(4/15)。 结论 局部晚期食管癌患者行TP方案诱导化疗+DDP同期放化疗的毒性可以耐受,局部失败仍然是主要的失败模式。 Abstract:Objective To assess the safety and efficacy of induction chemotherapy with cisplatin and docetaxel followed by radiation concurrent with weekly cisplatin for unresectable, locally advanced esophageal cancer. Methods Thirty-three patients with T3N0M0 to T4N2M0 thoracic esophageal squamous cell carcinoma without celiac lymph node metastasis were included in the study. They were treated with cisplatin (75 mg/m2 d1, d22) and docetaxel (75 mg/m2 d1, d22) neoadjuvant chemotherapy followed by three-dimensional conformal radiotherapy (60Gy/30F/6w) concurrent with cisplatin (30 mg/m2 d1, 8, 15, 22, 29, 36 from the beginning of radiation). Results Grade 4 hematological toxicities were observed in 13.33% (4/33) of the patients after the neoadjuvant chemotherapy. No grade 3 or above hepatic or renal toxicities were found. During concurrent chemoradiation, the highest grade 3 hematological toxicities were observed in the erythrocyte, granulocyte, and macrophage at 21.21% (7/33), 15.15% (5/33), and 3.01% (1/33), respectively. No grade 2 or above hepatic or renal toxicities were observed. Grade 3 radiation esophagitis was observed in 9.1% (3/33) of the patients, whereas grade 3 and above radiation esophagitis or grade 1 and above acute radiation pneumonitis did not occur. The evaluation results after treatment completion were 84.85% (28/33), 12.12% (4/33), and 3.03% (1/33) for CR+PR, SD, and PD, respectively. Two months after treatment completion, the results changed to 75.76% (25/33), 9.10% (3/33), and 15.15% (5/33), respectively. Overall, 15 patients died. The one-year survival rate was 66.4%. Local failure was approximately 46.67% (7/15), whereas the local+distant failure was approximately 26.67% (4/15). Therefore, local failure is the main pattern of failure in esophageal cancer. Conclusion The results indicate that neoadjuvant chemotherapy with cisplatin and docetaxel followed by radiotherapy concurrent with weekly cisplatin for locally advanced esophageal cancer is safe. Local failure remains the main pattern of failure in esophageal cancer. -
表 1 36例患者一般临床资料
Table 1. Patient characteristics

表 2 33例食管癌患者诱导化疗的毒性
Table 2. Toxicities of neoadjuvant chemotherapy

表 3 33例食管癌患者同期放化疗的毒性
Table 3. Toxicities of concurrent chemotherapy

-
[1] Chen CZ, Chen JZ, Li DR, et al. Long-term outcomes and prognostic factors for patients with esophageal cancer following radiotherapy[J]. World J Gastroenterol, 2013, 19(10): 1639-1644. doi: 10.3748/wjg.v19.i10.1639 [2] Chen L, Hu CS, Chen XZ, et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial[J]. Lancet Oncol, 2012, 13(2): 163-171. doi: 10.1016/S1470-2045(11)70320-5 [3] Hanna N, Neubauer M, Yiannoutsos C, et al. Phase Ⅲ study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage Ⅲ non-small-cell lung cancer: the Hoosier Oncology Group and U. S. Oncology[J]. J Clin Oncol, 2008, 26(35): 5755-5760. doi: 10.1200/JCO.2008.17.7840 [4] Tawfik HA, Taha Ael-H, Attia GA. Induction docetaxel and cispla tin followed by weekly docetaxel and cisplatin with concurrent radiotherapy in locally advanced stage Ⅲ non small cell lung cancer (LA-NSCLC)-a phase Ⅱ study[J]. J Egypt Natl Canc Inst, 2007, 19 (1): 15-20. [5] Scagliotti GV, Szczesna A, Ramlau R, et al. Docetaxel-based induction therapy prior to radiotherapy with or without docetaxel for non-small-cell lung cancer[J]. Br J Cancer, 2006, 94(10): 1375-1382. doi: 10.1038/sj.bjc.6603115 [6] Lin CC, Hsu CH, Cheng JC, et al. Concurrent chemoradiotherapy with twice weekly paclitaxel and cisplatin followed by esophagectomy for locally advanced esophageal cancer[J]. Ann Oncol, 2007, 18: 93-98. doi: 10.1093/annonc/mdl339 [7] Knox J, Wong R, Visbal AL. Phase 2 trial of preoperative irinotecan plus cisplatin and conformal radiotherapy, followed by surgery for esophageal cancer[J]. Cancer, 116(17): 4023-4032. doi: 10.1002/cncr.25349 [8] 中国非手术治疗食管癌临床分期专家小组. 非手术治疗食管癌的临床分期标准[J]. 中华放射肿瘤学杂志, 2010, 19(3): 179-180. doi: 10.3760/cma.j.issn.1004-4221.2010.03.001 [9] Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase Ⅲ trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy[J]. J Clin Oncol, 2002, 20(5): 1167-1174. doi: 10.1200/JCO.2002.20.5.1167 [10] Hsu FM, Chengl JC, Lin CC, et al. Esophageal squamous cell carcinoma treated by definitive or neoadjuvant chemoradiotherapy with or without paclitaxel[J]. Int J Radiat Oncol Biol Phys, 2006, 66 suppl 3: 278. [11] 李巧巧, 胡永红, 刘孟忠, 等. 放疗同期多西紫杉醇和顺铂化疗不能手术食管癌的疗效观察[J]. 中华放射肿瘤学杂志, 2009, 18(5): 375-378. doi: 10.3760/cma.j.issn.1004-4221.2009.05.375 [12] Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group[J]. JAMA, 1999, 281(17): 1623-1627. [13] 王谨, 庄婷婷, 何智纯, 等. 非小细胞肺癌同期放化疗后急性放射性肺炎的预测因素[J]. 中华放射肿瘤学杂志, 2012, 21(4): 326-329. doi: 10.3760/cma.j.issn.1004-4221.2012.04.011 -




 下载:
下载: