PhaseⅠStudy of nimotuzumab combined with postoperative chemoradiotherapy in Chinese patients with malignant glioma
-
摘要:
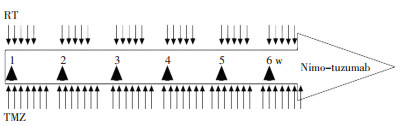
目的 恶性胶质瘤的预后较差,急需探索新的治疗策略以提高疗效。尼妥珠单抗是一种人源化抗表皮生长因子受体的单克隆抗体,能够抑制肿瘤细胞增殖,在一些恶性胶质瘤的Ⅰ/Ⅱ期临床研究中显示出了良好的耐受性及有效性。为此进行Ⅰ期临床试验,观察尼妥珠单抗联合术后同步放化疗治疗恶性胶质瘤的药物不良反应,耐受剂量及临床可行性。 方法 选取经病理确诊的Ⅲ~Ⅳ级脑胶质瘤术后患者为研究对象,采用术后替莫唑胺同步放化疗的标准治疗方案加尼妥珠单抗靶向治疗。尼妥珠单抗分100、200、400 mg/周3个剂量级,每剂量组3~6例,从低剂量组开始用药,如3例均未出现3级以上不良反应则进入下一剂量组。尼妥珠单抗采用静脉滴注,放疗期间1次/周,共6次。 结果 共有9例恶性胶质瘤入组,其中Ⅲ级胶质瘤7例,Ⅳ级2例。治疗过程中出现的不良反应均为1~2级,骨髓抑制为最常见的不良反应。临床治疗剂量达到400 mg/周时并未出现3级以上不良反应,可良好耐受。3个月后评价近期疗效,5例病情稳定,4例出现进展。 结论 本研究显示尼妥珠单抗联合术后同步放化疗对于治疗华人恶性胶质瘤的不良反应轻微,可良好耐受。 Abstract:Objective The poor prognosis of patients with malignant gliomas (MG) has led to the search for new therapeutic strategies. Recently, nimotuzumab has been studied as a new anti-EGFR-receptor humanized monoclonal antibody in patients with MG, who showed improvement of outcome and good tolerability. We conducted phase I of our study to determine the toxicity, tolerated dose, and clinical feasibility of nimotuzumab in combination with concurrent chemoradiotherapy for Chinese MG patients after surgical resection. Methods Patients with pathologically proven grades 3 and 4 glioma were enrolled in the study. The protocol included infusions of nimotuzumab plus standard Stupp schedule (postoperative radiotherapy in a total dose of 60 Gy in combination with daily temozolomide). Patients received 6 weekly infusions of nimotuzumab at three levels (100, 200, and 400 mg/week). If none of the first three patients enrolled at a dose level experienced dose-limiting toxicity (DLT), the dose was increased, as appropriate. If DLT was observed, another three patients were added to the dose level. Results Nine patients with MG were enrolled, including 7 with grade 3 MG and 2 with glioblastoma. The treatment was well tolerated, and no evidence of grade 3 or 4 adverse events was detected, even at the highest level (400 mg/week). Grade 1 or 2 myelosuppression was the most common toxicity. Three months after treatment, stable disease occurred in 5 patients, whereas progression disease was observed in 4 patients. Conclusion Nimotuzumab combined with concurrent chemoradiotherapy was associated with mild toxicity in Chinese MG patients. -
Key words:
- malignant glioma /
- EGFR /
- nimotuzumab /
- chemoradiotherapy
-
表 1 患者情况
Table 1. Patient characteristics

表 2 治疗期间主要不良事件分级(CTCAE3.0分级)
Table 2. Adverse events during treatment (grade of CTCAE 3.0)

-
[1] Hargrave DR, Zacharoulis S. Pediatric CNS tumors: current treatment and future directions[J]. Expert Rev Neurother, 2007, 7(8): 1029-1042. doi: 10.1586/14737175.7.8.1029 [2] Sarissky M, Lavicka J, Kocanova S, et al. Diazepam enhances hypericin-induced photocytotoxicity and apoptosis in human glioblastoma cells[J]. Neoplasma, 2005, 52(4): 352-359. [3] Hulsebos TJ, Troost D, Leenstra S. Molecular-genetic characterisation of gliomas that recur as same grade or higher grade tumours[J]. J Neurol Neurosurg Psychiatry, 2004, 75(5): 723-726. doi: 10.1136/jnnp.2003.025031 [4] Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase Ⅲ study 5-year analysis of the EORT C-NCIC trial[J]. Lancet Oncol, 2009, 10(5): 459-466. doi: 10.1016/S1470-2045(09)70025-7 [5] Herbst RS, Shin DM. Monoclonal antibodies to target epidermal growth factor receptor-positive tumors: a new paradigm for cancer therapy[J]. Cancer, 2002, 94(5): 1593-1611. doi: 10.1002/cncr.10372 [6] Nishikawa R, Sugiyama T, Narita Y, et al. Immunohistochemical analysis of the mutant epidermal growth factor, detla EGFR, in glioblastoma[J]. Brain tumor pathol, 2004, 21(2): 53-56. doi: 10.1007/BF02484510 [7] Casacó A, López G, García I, et al. Phase I single-dose study of intracavitary-administered Nimotuzumab labeled with 188 Re in adult recurrent high grade glioma[J]. Cancer Biol Ther, 2008, 7(3): 333-339. doi: 10.4161/cbt.7.3.5414 [8] Ramos TC, Figueredo J, Catala M, et al. Treatment of high-grade glioma patients with the humanized anti-epidermal growth factor receptor (EGFR) antibody h-R3: report from a phaseⅠ/Ⅱtrial[J]. Cancer biol ther, 2006, 5(4): 375-379. doi: 10.4161/cbt.5.4.2522 [9] You B, Brade A, Magalhaes JM, et al. A dose-escalation phase I trial of nimotuzumab, an antibody against the epidermal growth factor receptor, in patients with advanced solid malignancies[J]. Invest New Drugs, 2011, 29(5): 996-1003. doi: 10.1007/s10637-010-9444-0 [10] Bode U, Massimino M, Bach F, et al. Nimotuzumab treatment of malignant gliomas[J]. Expert opin Biol Ther, 2012, 12(12): 1649-1659. doi: 10.1517/14712598.2012.733367 [11] Akashi Y, Okamoto I, Iwasa T, et al. Enhancement of the antitumor activity of ionising radiation by nimotuzumab, a humanised monoclonal antibody to the epidermal growth factor receptor, in non-small cell lung cancer cell lines of differing epidermal growth factor receptor status[J]. Br J Cancer, 2008, 98(4): 749-755. doi: 10.1038/sj.bjc.6604222 [12] Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer[J]. JAMA, 1999, 281(17): 1623-1627. [13] Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase Ⅲ trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy[J]. J Clin Oncol, 2002, 20(5): 1167-1174. doi: 10.1200/JCO.2002.20.5.1167 [14] Crombet T, Osorio M, Cruz T, et al. Use of the humanized anti-epidermal growth factor receptor monoclonal antibody h-R3 in combination with radiotherapy in the treatment of locally advanced head and neck cancer patients[J]. J Clin Oncol, 2004, 22(9): 1646-1654. doi: 10.1200/JCO.2004.03.089 [15] Crombet T, Torres L, Neninger E, et al. Pharmacological evaluation of humanized anti-epidermal growth factor receptor, monoclonal antibody h-R3, in patients with advanced epithelial-derived cancer[J]. J Immu nother, 2003, 26(2): 139-148. [16] Dragovich T, Campen C. Anti-EGFR-targeted therapy for esophageal and gastric cancers: an evolving concept[J]. J Oncol, 2009: 804108. [17] Ramos TC, Figueredo J, Catala M, et al. Treatment of high-grade glioma patients with the humanized anti-epidermal growth factor receptor (EGFR) antibody h-R3: report from a phase Ⅰ/Ⅱ trial[J]. Canc Biol Ther, 2006, 5(4): 375-379. doi: 10.4161/cbt.5.4.2522 [18] Lam C, Bouffet E, Bartels U. Nimotuzumab in pediatric glioma[J]. Future Oncol, 2009, 5(9): 1349-1361. doi: 10.2217/fon.09.119 [19] Miqueli AD, Rolff J, Lemm M, et al. Radiosensitisation of U87MG brain tumours by anti-epidermal growth factor receptor monoclonal antibodies[J]. Br J Cancer, 2009, 100(6): 950-958. doi: 10.1038/sj.bjc.6604943 [20] Harari PM. Epidermal growth factor receptor inhibition strategies in oncology[J]. Endocr Relat Cancer, 2004, 11(4): 689-708. doi: 10.1677/erc.1.00600 [21] Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phaseⅢ trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy[J]. J Clin Oncol, 2002, 20(5): 1167-1174. doi: 10.1200/JCO.2002.20.5.1167 -




 下载:
下载: