Clinical features and risk factors of death within the first chemotherapy cycle in multiple myeloma
-
摘要:
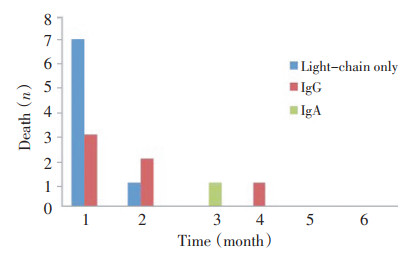
目的 探讨多发性骨髓瘤(MM)发生初次化疗近期死亡患者的临床特征和危险因素。 方法 对2004年1月~2012年10月期间初治的111例MM患者的临床资料进行回顾性分析。 结果 MM患者确诊后6个月内死亡15例,其中10例于第1个化疗用药期或间歇期死亡,占全部化疗例数的9%。10例初次化疗近期死亡患者,中位生存时间为5天。其中,轻链型7例,IgG型3例;死于严重感染及并发症8例。单因素分析显示,IFE分型为轻链型、化疗前低血小板、低白细胞、低中性粒细胞水平、ECOG评分升高、血清肌酐水平升高与第1个周期化疗近期死亡风险增加有关。多因素回归分析显示,M蛋白为轻链型(P=0.003,OR= 12.976,95%CI:2.328~72.322)、化疗前血小板低水平(P=0.034,OR=6.141,95%CI:1.152~32.739)及中性粒细胞水平(P=0.003,OR=13.639,95%CI:2.398~77.564)是发生初次化疗近期死亡的独立危险因素。 结论 初始化疗对于相当部分MM患者具有特殊的致命风险。严重感染及其并发症是直接导致化疗近期死亡的主要原因。影响MM总体生存的预后因素并不能完全决定初次化疗的死亡风险。轻链型骨髓瘤、化疗前低血小板及低中性粒细胞水平是发生初次化疗近期死亡的独立危险因素。 Abstract:Objective To study the clinical features and risk factors of death within the first chemotherapy cycle in multiple myeloma (MM). Methods From 2004 to 2012, 111 patients with MM received first-line chemotherapy. Their clinical data were recorded and analyzed retrospectively. Results A total of 15 patients died within six months after diagnosis, whereas 10 patients (9%) died within the first chemotherapy cycle. The median overall survival was five days. The monoclonal protein isotype of 10 patients was as follows: light chain only, 7; IgG, 3.8 of 10 cases of deaths were attributed to infection and complications. Univariate analysis revealed that light-chain myeloma, thrombocytopenia, leukopenia, neutropenia before treatment, high ECOG score, and high serum creatinine were predictors of death within the first chemotherapy cycle. Logistic regression modeling identified light-chain myeloma (P=0.003, OR 12.976, 95% CI: 2.328 to 72.322), thrombocytopenia (P=0.034, OR 6.141, 95% CI: 1.152 to 32.739), and neutropenia (P=0.003, OR 13.639, 95% CI: 2.398 to 77.564) as independent predictors. Conclusion Fatal risk could be elevated by the first cycle of chemotherapy in some patients with MM. The causes of death within the first chemotherapy cycle were mainly serious infection and complications. The prognostic factors of MM could not effectively predict the mortality risk of induction chemotherapy. Light-chain myeloma, thrombocytopenia, and neutropenia were independent predictors of death within the first chemotherapy cycle. -
Key words:
- multiple myeloma /
- death /
- risk factor
-
表 1 单因素分析第1个周期化疗近期死亡影响因素
Table 1. Univariate analysis of risk factors for death within the first chemotherapy cycle

表 2 多因素logistic回归分析第1个周期化疗近期死亡影响因素
Table 2. Logistic regression of risk factors for death within the first chemotherapy cycle

-
[1] Anderson KC. New insights into therapeutic targets in myeloma[A]. Hematology Am Soc Hematol Educ Program, 2011: 184-190. [2] Augustson BM, Begum G, Dunn JA, et al. Early Mortality After Diagnosis of Multiple Myeloma: Analysis of Patients Entered Onto the United Kingdom Medical Research Council Trials Between 1980 and 2002—Medical Research Council Adult Leukaemia Working Party[J]. J Clin Oncol, 2005, 23(36):9219-9226. doi: 10.1200/jco.2005.03.2086 [3] 张之南, 沈悌.血液病诊断及疗效标准[M].第2版.北京:科学出版社, 1998:373-380.Zhang Z, Shen T. Multiple myeloma Criteria of Diagnosis and Therapeutic Effect for Hematopathy[M]. 2nd edition. Science Press: Beijing, 1998:373-380. [4] Beksac M, Haznedar R, Firatli-Tuglular T, et al. Addition of thalidomide to oral melphalan/prednisone in patients with multiple myeloma not eligible for transplantation: results of a randomized trial from the Turkish Myeloma Study Group[J]. Eur J Haematol, 2011, 86(1):16-22. http://www.ncbi.nlm.nih.gov/pubmed/20942865 [5] Bladé J, Rosiñol L.Renal, hematologic and infectious complications in multiple myeloma[J]. Best Pract Res Clin Haematol, 2005, 18(4): 635-652. http://europepmc.org/abstract/med/16026742 [6] Murakami H, Hayashi K, Hatsumi N, et al. Risk factors for early death in patients undergoing treatment for multiple myeloma[J]. Ann Hematol, 2001, 80:452-455. doi: 10.1007/s002770100330 [7] Drayson M, Begum G, Basu S, et al. Effects of paraprotein heavy and light chain types and free light chain load on survival in myeloma: an analysis of patients receiving conventional-dose chemotherapy in Medical Research Council UK multiple myeloma trials[J]. Blood, 2006, 108(6):2013-2019. doi: 10.1182/blood-2006-03-008953 [8] Ludwig H, Hajek R, Tóthová E, et al. Thalidomide-dexamethasone compared with melphalan-prednisolone in elderly patients with multiple myeloma[J]. Blood, 2009, 113(15):3435-3442. http://europepmc.org/abstract/MED/18955563 [9] McCloskey EV, MacLennan IC, Drayson MT, et al. A randomized trial of the effect of clodronate on skeletal morbidity in multiple myeloma:MRC Working Party on Leukaemia in Adults[J]. Br J Haematol, 1998, 100(2):317-325. http://europepmc.org/abstract/MED/9488619 [10] Muthu Raja KR, Kubiczkova L, Rihova L, et al. Functionally Suppressive CD8 T Regulatory Cells Are Increased in Patients with Multiple Myeloma: A Cause for Immune Impairment[J]. PLoS One, 2012, 7(11):e49446. http://europepmc.org/articles/PMC3496705/ [11] Feyler S, Selby PJ, Cook G. Regulating the regulators in cancer-immunosuppression in multiple myeloma (MM)[J]. Blood Rev, 2013, 27(3):155-164. http://www.sciencedirect.com/science/article/pii/S0268960X13000209 [12] Palumbo A, Hajek R, Delforge M, et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma[J]. N Engl J Med, 2012, 366(19):1759-1769. http://europepmc.org/abstract/med/22571200 [13] Moreau P, Avet-Loiseau H, Facon T, et al. Bortezomib plus dexamethasone versus reduced-dose bortezomib, thalidomide plus dexamethasone as induction treatment before autologous stem cell transplantation in newly diagnosed multiple myeloma[J]. Blood, 2011, 118(22):5752-5758. http://www.ncbi.nlm.nih.gov/pubmed/21849487 [14] Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients[J]. Leukemia, 2013. doi: 10.10381len.2013.313.[Epub ahead of print] [15] Palumbo A, Bringhen S, Ludwig H, et al. Personalized therapy in multiple myeloma according to patient age and vulnerability: a report of the European Myeloma Network (EMN)[J]. Blood, 2011, 118(17):4519-4529. http://europepmc.org/abstract/MED/21841166 -




 下载:
下载: