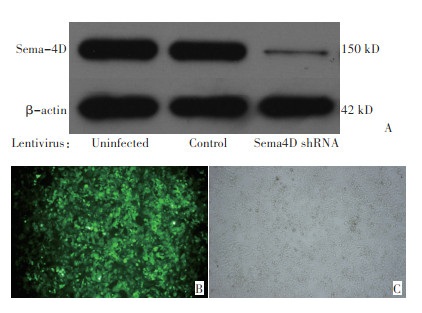
Sema4D deficiency reduces colorectal carcinoma xenograft growth and vascularity in nude mice
-
摘要:
目的 本文利用慢病毒介导的RNA干扰,在结直肠癌裸鼠皮下移植瘤模型中研究信号素4D(Sema4D) 对血管新生的影响。 方法 包装制备对照慢病毒和Sema4D shRNA干扰慢病毒,感染直肠癌细胞HCT-116。Transwell迁移实验检测对照组和干扰组诱导内皮细胞迁移的能力。对照组和干扰组分别接种于裸鼠双侧腋窝皮下。观察记录肿瘤生长,收获移植瘤做免疫组化和免疫荧光检。 结果 研究发现干扰Sema4D的表达能减弱癌细胞诱导内皮细胞迁移的能力,显著减缓移植瘤生长速度并降低瘤内血管密度。 结论 由于Sema4D在诱导血管新生中的作用,干扰其信号通路将可能通过抑制血管新生来阻止肿瘤生长或转移,因此Sema4D有可能成为肿瘤抗血管新生疗法中有价值的治疗靶点。 Abstract:Objective: Semaphorin 4D (Sema4D) acts as a regulator for axon guidance in central nervous system development.However, new evidence indicates that Sema4D has a previously unrecognized function, namely, compensatory angiogenic factor. Thisstudy aimed to investigate the effect of Sema4D on tumor growth and vascularity of colorectal carcinoma (CRC) in nude mice. Methods Sema4D was knocked down in CRC cells by infecting the cells with lentiviruses coding for Sema4D shRNA. Two groups of cells, namely, those infected with control viruses and those infected with Sema4D shRNA viruses, were subjected to migration assay to testtheir ability induce endothelial cell migration. The two cell groups were subcutaneously injected into nude mice. Tumor growth was documented, and the tumors harvested from the mice were subjected to immunohistochemistry or immunofluorescence analyses. Results In vitro migration assay results indicated that media conditioned by HCT-116 cells infected with Sema4D shRNA lentiviruses induced low endothelial cell migration. The two groups of subcutaneously inoculated cells showed 100% tumorigenicity. However, tumor growth rates were significantly different between the two groups. Xenografts in which Sema4D was downregulated showed marked reduction in tumor size and vascularity. Conclusion Cancer cells may highly express Sema4D to trigger net neo-angiogenesis and generate a tumor blood supply system. Thus, Sema4D could potentially be a target in anti-angiogenic therapy of CRC patients. -
Key words:
- colorectal carcinoma /
- semaphorin 4D /
- angiogenesis /
- xenografts /
- RNAi
-
图 2 Transwell迁移实验(×100)
Figure 2. Migration assay
Positive control,Dulbecco‘s modi fi ed Eagle’s medium (DMEM)with 10% fetal bovine serum(A). Negative control,serum-free DMEM(B). Conditioned serum-free DMEM from cells infected with the control virus (C).Conditioned serum-free DMEM from cells infected with Sema4D shRNA lentivirus(D). Human umbilical vein endothelial cell relative migration rate(E)
图 5 免疫组织化学检测2组移植瘤中Sema4D的表达(×200)及免疫荧光检测血管密度(×200)
Figure 5. Immunohistochemistry analysis for Sema4D and immunofluorescence for vascular density×200
Tumors from infected control cells were stained for Sema4D(A)and endothelial cells(CD31)(C). Tumor cells expressing SEMA4D shRNA were stained for Sema4D(B)and CD31(D). Relative fluorescence intensities(E)
-
[1] Hoffman RM, Espey DK, Rhyne RL, et al. Colorectal cancer inci dence and mortality disparities in new Mexico[J]. J Cancer Epidemiol, 2014, Epub2014 Jan 2. [2] Kekelidze M, D'Errico L, Pansini M, et al. Colorectal cancer: Cur rent imaging methods and future perspectives for the diagnosis, staging and therapeutic response evaluation[J]. World J Gastroenterol, 2013, 19(46):8502-8514. doi: 10.3748/wjg.v19.i46.8502 [3] Kokki I, Papana A, Campbell H, et al. Estimating the incidence of colorectal cancer in South East Asia[J]. Croat Med J, 2013, 54(6): 532-540. doi: 10.3325/cmj.2013.54.532 [4] Nimptsch K, Malik VS, Fung TT, et al. Dietary patterns during high school and risk of colorectal adenoma in a cohort of mid dle-aged women[J]. Int J Cancer, 2014, 134(10):2458-2467. doi: 10.1002/ijc.28578 [5] Bellou S, Pentheroudakis G, Murphy C, et al. Anti-angiogenesis in cancer therapy: Hercules and hydra[J]. Cancer Lett, 2013, 338(2): 219-228. doi: 10.1016/j.canlet.2013.05.015 [6] Ellis LM, Hicklin DJ. Pathways mediating resistance to vascular en dothelial growth factor-targeted therapy[J]. Clin Cancer Res, 2008, 14(20):6371-6375. doi: 10.1158/1078-0432.CCR-07-5287 [7] Casanovas O, Hicklin DJ, Bergers G, et al. Drug resistance by eva sion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors[J]. Cancer Cell, 2005, 8(4):299-309. doi: 10.1016/j.ccr.2005.09.005 [8] Basile JR, Barac A, Zhu T, et al. Class IV semaphorins promote an giogenesis by stimulating Rho-initiated pathways through plexin-B [J]. Cancer Res, 2004, 64(15):5212-5224. doi: 10.1158/0008-5472.CAN-04-0126 [9] Basile JR, Castilho RM, Williams VP, et al. Semaphorin 4D provides a link between axon guidance processes and tumor-induced angio genesis[J]. Proc Natl Acad Sci U S A, 2006, 103(24):9017-9022. doi: 10.1073/pnas.0508825103 [10] Basile JR, Holmbeck K, Bugge TH, et al. MT1-MMP controls tu mor-induced angiogenesis through the release of semaphorin 4D [J]. J Biol Chem, 2007, 282(9):6899-6905. doi: 10.1074/jbc.M609570200 [11] Pastushenko I, Vermeulen PB, Carapeto FJ, et al. Blood microvessel density, lymphatic microvessel density and lymphatic invasion in predicting melanoma metastases: systematic review and meta-anal ysis[J]. Br J Dermatol, 2014, 170(1):66-77. https://europepmc.org/article/PMC/PMC6528563 [12] Gerger A, LaBonte M, Lenz HJ. Molecular predictors of response to antiangiogenic therapies[J]. Cancer J, 2011, 17(2):134-141. doi: 10.1097/PPO.0b013e318212db3c [13] Jain RK, Duda DG, Clark JW, et al. Lessons from phase III clinical trials on anti-VEGF therapy for cancer[J]. Nat Clin Pract Oncol, 2006, 3(1):24-40. doi: 10.1038/ncponc0403 [14] Moserle L, Casanovas O. Anti-angiogenesis and metastasis: a tu mour and stromal cell alliance[J]. J Intern Med, 2013, 273(2): 128-137. https://dialnet.unirioja.es/servlet/articulo?codigo=5987596 [15] Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system[J]. Nat Med, 2003, 9(6):677-684. doi: 10.1038/nm0603-677 [16] Shibuya M, Claesson-Welsh L. Signal transduction by VEGF re ceptors in regulation of angiogenesis and lymphangiogenesis[J]. Exp Cell Res, 2006, 312(5):549-560. doi: 10.1016/j.yexcr.2005.11.012 [17] Mizumaki Y, Jo WS, Duerr EM, et al. Induction of interleukin-8 preserves the angiogenic response in HIF-1 alpha-deficient colon cancer cells[J]. Nat Rev, 2005, 11(9):992-997. https://www.nature.com/articles/nm1294 [18] Zhou H, Yang YH, Binmadi NO, et al. The hypoxia-inducible fac tor-responsive proteins semaphorin 4D and vascular endothelial growth factor promote tumor growth and angiogenesis in oral squa mous cell carcinoma[J]. Exp Cell Res, 2012, 318(14):1685-1698. doi: 10.1016/j.yexcr.2012.04.019 [19] Zhou H, Binmadi NO, Yang YH, et al. Semaphorin 4D cooperates with VEGF to promote angiogenesis and tumor progression[J]. An giogenesis, 2012, 15(3):391-407. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3733222/ -




 下载:
下载: