p-STAT3 promotes colorectal cancer metastasis by inducing epithelial-mesenchymal transition
-
摘要:
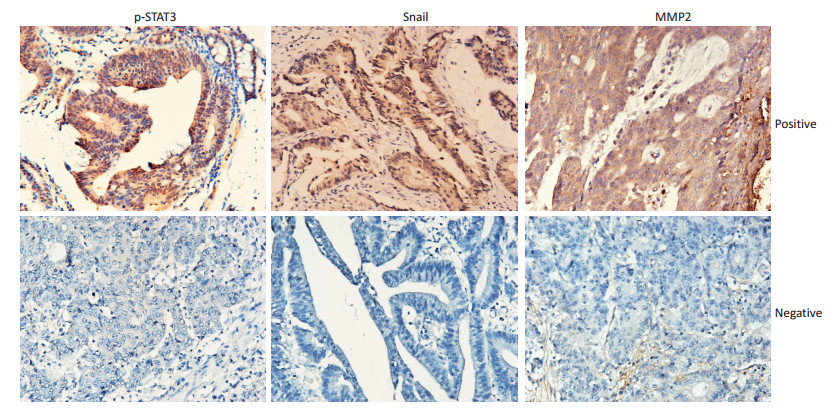
目的 研究p-STAT3的活化在结肠癌中的表达与Snail、MMP2的相关性,及其在离体细胞实验中激活受抑后对结肠癌细胞迁移能力的影响并探讨其机制。 方法 免疫组织化学染色检测p-STAT3与Snail、MMP2的表达及其相关性,分析三者与TNM分期、远处转移、淋巴结转移及分化程度之间的关系;MTT实验筛选AG490对增殖无影响的浓度和时间,并用该浓度和时间进行后续实验;采用Western blot法检测AG490抑制p-STAT3活化后STAT3、Snail、MMP2蛋白表达情况;划痕实验观察p-STAT3活化受抑后,结肠癌细胞迁移情况。 结果 免疫组织化学染色结果表明,p-STAT3的表达与Snail、MMP2均存在相关性,且均与淋巴结转移相关(P < 0.05)。在两个结肠癌细胞系中,通过MTT实验,选出10 μM作为AG490的最佳作用浓度,并采用该浓度进行后续实验。AG490抑制p-STAT3活化后Snail、MMP2表达明显下降,而STAT3总蛋白表达无明显变化。且当AG490抑制p-STAT3活化后,细胞的迁移能力明显下降。 结论 p-STAT3可以通过上皮间质转化(epithelial-mesenchymal transition,EMT)促进结肠癌的转移。 Abstract:Objective To determine p-STAT3 expression in 65 cases of colorectal cancer and its correlation with Snail and MMP2 and to explore the mechanism of p-STAT3 activation in colorectal cancer cells by inhibiting its activation in vitro. Methods Immunohistochemical technique was adopted to examine the expression levels of p-STAT3, Snail, and MMP2. MTT assay was used to explore the effects of diluting AG490, which does not affect cell proliferation. Western blot was used to examine the expression levels of p-STAT3, STAT3, Snail, and MMP2. Wound healing assay was employed to evaluate the migration of colorectal cancer cells. Results p-STAT3 expression was significantly correlated with Snail and MMP2 expression. Moreover, p-STAT3 expression was significantly correlated with TNM stage, lymph node metastasis, and distant metastasis in colorectal cancer tissues (P < 0.05) but not with tumor differentiation. Treatment with 10 μM AG490 for 48 h did not significantly affect cell proliferation in the control and experimental groups (P > 0.05). STAT3 expression was not significantly changed when p-STAT3 expression was inhibited by AG490. Meanwhile, the expression levels of Snail and MMP2 were down-regulated, and the migration ability of colorectal cancer cells was significantly reduced. Conclusion p-STAT3 promotes colorectal cancer metastasis by regulating the process of epithelial-mesenchymal transition. -
Key words:
- colorectal cancer /
- epithelial-mesenchymal transition /
- p-STAT3 /
- Snail /
- MMP2 /
- AG490
-
图 2 各浓度AG490对结肠癌细胞的生长抑制作用
Figure 2. Effects of different AG490 concentrations on colorectal cancer cell lines
A. HCT116 cell line, B. HT29 cell line; a: P < 0.05, compared with the control group at the same time; b: Compared with the same concentration at 24 h; c: Compared with the same concentration at 48 h
图 4 AG490抑制p-STAT3活化对结肠癌细胞迁移能力的影响
Figure 4. Effects of AG490 treatment on the migration capacity of colorectal cell lines
A, B. Wound healing assay; C. Analysis of mobility in HCT116 cells after 0, 24, and 48 h of AG490 treatment; D: Analysis of mobility in HT29 cells after 0, 24, and 48 h of AG490 treatment
表 1 p-STAT3与Snail、MMP2的相关性(n=65)
Table 1. Correlation among p-STAT3 and Snail as well as MMP2 (n=65)

表 2 p-STAT3、Snail、MMP2与TNM分期、淋巴结转移、远处转移及分化程度的相关性(n=65)
Table 2. Correlation of p-STAT3, Snail and MMP2 with TNM stage, lymph node metastasis, distant metastasis, and differentiation (n=65)

-
[1] Merikallio H, Turpeenniemi-Hujanen T, Pääkkö P, et al. Snail promotes an invasive phenotype in lung carcinoma[J]. Respir Res, 2012, 13:104. doi: 10.1186/1465-9921-13-104 [2] Cao LY, Yang J, Fu XG, et al. The MicroRNA miR-205 inhibits epithelial-messenchymal transition in HK-2 cells by down-regulating ZEB1 and ZEB2 expressions[J]. Nan Fang Yi Ke Da Xue Xue Bao, 2016, 36 (12):1700-1705. https://www.ncbi.nlm.nih.gov/pubmed/27998868 [3] Li W, Wang Z, Zha L, et al. HMGA2 regulates epithelial-mesenchymal transition and the acquisition of tumor stem cell properties through TWIST1 in gastric cancer[J]. Oncol Rep, 2017, 37(1):185-192. https://www.ncbi.nlm.nih.gov/pubmed/27878307 [4] Hajimoradi M, Mohammad HZ, Ebrahimi M, et al. STAT3 is overactivated in gastric cancer stem-like cells[J]. Cell J, 2016, 17(4):617-628. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4746412/ [5] Mao W, Sun Y, Zhang H, et al. A combined modality of carboplatin and photodynamic therapy suppresses epithelial-mesenchymal transition and matrix metalloproteinase-2 (MMP-2)/MMP-9 expression in HEp-2 human laryngeal cancer cells via ROS-mediated inhibition of MEK/ERK signalling pathway[J]. Lasers Med Sci, 2016, 31(8):1697-1705. doi: 10.1007/s10103-016-2040-6 [6] Bittner M, Meltzer P, Chen Y, et al. Molecular classification of cutaneous malignant melanoma by gene expression profiling[J]. Nature, 2000, 406(6795):536-540. doi: 10.1038/35020115 [7] 吕强, 邢沈阳, 赵志辉, 等.结肠癌的研究现状及展望[J].中国实验诊断学, 2009, (8):1134-1137. http://www.cnki.com.cn/Article/CJFDTOTAL-ZSZD200908057.htmLv Q, Xing SY, Zhao ZH, et al. Research and prospect of colon cancer [J]. Chin J Labor Diag, 2009, (8):1134-1137. http://www.cnki.com.cn/Article/CJFDTOTAL-ZSZD200908057.htm [8] Aleskandarany MA, Agarwal D, Negm OH, et al. The prognostic significance of STAT3 in invasive breast cancer: analysis of protein and mRNA expressions in large cohorts[J]. Breast Cancer Res Treat, 2016, 156(1):9-20. doi: 10.1007/s10549-016-3709-z [9] Song YY, Sun LD, Liu ML, et al. STAT3, p-STAT3 and HIF-1α are associated with vasculogenic mimicry and impact on survival in gastric adenocarcinoma[J]. Oncol Lett, 2014, 8(1):431-437. https://www.spandidos-publications.com/ol/8/1/431/abstract [10] Suiqing C, Min Z, Lirong C. Overexpression of phosphorylated-STAT3 correlated with the invasion and metastasis of cutaneous squamous cell carcinoma[J]. J Dermatol, 2005, 32(5):354-360. doi: 10.1111/jde.2005.32.issue-5 [11] Cui Y, Li YY, Li J, et al. STAT3 regulates hypoxia-induced epithelial mesenchymal transition in oesophageal squamous cell cancer[J]. Oncol Rep, 2016, 36(1):108-116. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4899013/ [12] Li X, Bhaduri-McIntosh S. A central role for STAT3 in gammaherpesvirus-Life cycle and -diseases[J]. Front Microbiol, 2016, 7:1052. [13] Haura EB, Turkson J, Jove R. Mechanisms of disease: Insights into the emerging role of signal transducers and activators of transcription in cancer[J]. Nat Clin Pract Oncol, 2005, 2(6):315-324. doi: 10.1038/ncponc0195 [14] 张晓芹, 史迎莉, 贾小云, 等.p-STAT3与缺氧诱导因子在结肠癌组织中的表达及其临床意义[J].中国老年学杂志, 2014, (15):4121-4123. doi: 10.3969/j.issn.1005-9202.2014.15.003Zhang XQ, Shi YL, Jia XY, et al. Expression and clinical significance of p-STAT3 and hypoxia inducible factor in colon cancer[J]. Chin J Geront, 2014, (15):4121-4123. doi: 10.3969/j.issn.1005-9202.2014.15.003 [15] Liu J, Ben QW, Yao WY, et al. BMP2 induces PANC-1 cell invasion by MMP-2 overexpression through ROS and ERK[J]. Front Biosci (Landmark Ed), 2012, 17:2541-2549. doi: 10.2741/4069 [16] Li CH, Zhao JX, Sun L, et al. AG490 inhibits NFATc1 expression and STAT3 activation during RANKL induced osteoclastogenesis[J]. Biochem Biophys Res Commun, 2013, 435(4):533-539. doi: 10.1016/j.bbrc.2013.04.084 [17] Zhou JJ, Meng Z, He XY, et al. Hepatitis C virus core protein increases Snail expression and induces epithelial-mesenchymal transition through the signal transducer and activator of transcription 3 pathway in hepatoma cells[J]. Hepatol Res, 2017, 47(6):574-583. doi: 10.1111/hepr.v47.6 [18] 杨光, 裘正军, 张放, 等.激活和抑制Stat3信号途径对胰腺癌细胞侵袭能力的影响[J].肿瘤, 2009, (7):645-649. http://www.cnki.com.cn/Article/CJFDTOTAL-ZZLL200907012.htmYang G, Qiu ZJ, Zhang F, et al. Effect sofactivation and inhibition of Stat3 signaling pathway on invasion of human pancreatic cancer cell[J]. Tumor, 2009, (7):645-649. http://www.cnki.com.cn/Article/CJFDTOTAL-ZZLL200907012.htm -




 下载:
下载: