Advances of chimeric antigen receptor T-cell immunotherapy combined with immune checkpoint inhibitors in malignancies therapy
-
摘要: 近年来,嵌合抗原受体T细胞(chimeric antigen receptor T-cell,CAR-T)治疗在恶性肿瘤治疗中取得了较多成果,尤其在血液肿瘤治疗方面有所突破,实体瘤治疗研究前景较为广阔,有望成为更多复发难治性肿瘤患者的选择。免疫检查点抑制剂疗法在肿瘤治疗中同样具有疗效,如肝癌、难治性霍奇金淋巴瘤等多种恶性肿瘤,为晚期肿瘤患者带来了希望。但是上述两种治疗方法均存在不同程度的局限性。CAR-T联合免疫检查点抑制剂会削弱肿瘤微环境的免疫抑制作用,提高CAR-T治疗的有效率,延长生存期。本文旨在对CAR-T细胞和免疫检查点抑制剂治疗及二者联合应用的研究进展予以综述。Abstract: Recent years, chimeric antigen receptor T-cell (CAR-T) therapy has made great strides in enabling better treatment of malignant tumors, especially hematological tumors, as well as increasing the research prospects of potential solid tumor therapies. Consequently, CAR-T therapy is expected to become the selected treatment for patients with relapsed and refractory tumors. Immune checkpoint inhibitors also have certain curative effects in the treatment of cancers, such as liver cancer, refractory Hodgkin's lymphoma, and other malignant tumors, which brings promise to patients with advanced cancer. However, both treatments have different degrees of limitations. Recent clinical trials suggest that combined immune checkpoint inhibitors impair the immunosuppressive effects of the tumor microenvironment, increase the efficacy of CAR-T therapy, and prolong the survival. This article will review the research progress on the combination of CAR-T immunotherapy and immune checkpoint inhibitors in malignancies theray.
-
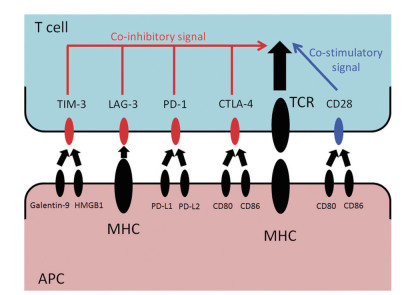
图 1 免疫检查点对T细胞的影响[22]
Galectin-9:天然型配体半乳糖凝集素9;HMGB1:高迁移率族蛋白1(Galectin-9和HMGB1均为TIM-3的配体);MHC:主要组织相容性复合体;TCR:T细胞受体;APC:抗原提呈细胞;T细胞主要的激活信号由TCR介导,共刺激信号由CD28提供。共刺激信号由PD-1、CTLA-4、LAG-3和TIM-3等抑制性信号调节
-
[1] Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia[J]. N Engl J Med, 2014, 371(16):1507-1517. doi: 10.1056/NEJMoa1407222 [2] Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-Cell therapy in refractory large B-cell lymphoma[J]. New Engl J Med, 2017, 377(26):2531-2544. doi: 10.1056/NEJMoa1707447 [3] Luo F, Qian J, Yang J, et al. Bifunctional alphaHER2/CD3 RNA-engineered CART-like human T cells specifically eliminate HER2(+) gastric cancer[J]. Cell Res, 2016, 26(7):850-853. doi: 10.1038/cr.2016.81 [4] Brown CE, Alizadeh D, Starr R, et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy[J]. N Engl J Med, 2016, 375(26):2561-2569. doi: 10.1056/NEJMoa1610497 [5] Xu-Monette ZY, Zhou J, Young KH. PD-1 expression and clinical PD-1 blockade in B-cell lymphomas[J]. Blood, 2018, 131(1):68-83. http://www.ncbi.nlm.nih.gov/pubmed/29118007 [6] Nghiem PT, Bhatia S, Lipson EJ, et al. PD-1 blockade with pembrolizumab in advanced merkel-cell carcinoma[J]. N Engl J Med, 2016, 374(26):2542-2552. doi: 10.1056/NEJMoa1603702 [7] Lim WA, June CH. The principles of engineering immune cells to treat cancer[J]. Cell, 2017, 168(4):724-740. doi: 10.1016/j.cell.2017.01.016 [8] Chmielewski M, Abken H. TRUCKs: the fourth generation of CARs [J]. Exp Opin Biol Ther, 2015, 15(8):1145-1154. doi: 10.1517/14712598.2015.1046430 [9] Tashiro H, Sauer T, Shum T, et al. Treatment of acute myeloid leukemia with T cells expressing chimeric antigen receptors directed to c-type lectin-like molecule 1[J]. Mol Ther, 2017, 25(9):2202-2213. doi: 10.1016/j.ymthe.2017.05.024 [10] Garfall AL, Maus MV, Hwang WT, et al. Chimeric antigen receptor T cells against CD19 for multiple myeloma[J]. N Engl J Med, 2015, 373 (11):1040-1047. doi: 10.1056/NEJMoa1504542 [11] Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma[J]. N Engl J Med, 2017, 377(26):2531-2544. doi: 10.1056/NEJMoa1707447 [12] Fry TJ, Shah NN, Orentas RJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy[J]. Nat Med, 2017, (24):20. https://www.researchgate.net/publication/321175475_CD22-targeted_CAR_T_cells_induce_remission_in_B-ALL_that_is_naive_or_resistant_to_CD19-targeted_CAR_immunotherapy [13] Mikkilineni L, Kochenderfer JN. Chimeric antigen receptor T-cell therapies for multiple myeloma[J]. Blood, 2017, 130(24):2594. doi: 10.1182/blood-2017-06-793869 [14] Wang J, Chen S, Xiao W, et al. CAR-T cells targeting CLL-1 as an approach to treat acute myeloid leukemia[J]. J Hematol Oncol, 2018, 11(1):7. doi: 10.1186/s13045-017-0553-5 [15] Arcangeli S, Rotiroti MC, Bardelli M, et al. Balance of anti-CD123 chimeric antigen receptor binding affinity and density for the targeting of acute myeloid leukemia[J]. Mol Ther, 2017, 25(8):1933-1945. doi: 10.1016/j.ymthe.2017.04.017 [16] Guo Y, Feng K, Liu Y, et al. PhaseⅠstudy of chimeric antigen receptor-modified T cells in patients with EGFR-positive advanced biliary tract cancers[J]. Clin Cancer Res, 2018, 24(6):1277. doi: 10.1158/1078-0432.CCR-17-0432 [17] Tchou J, Zhao Y, Levine BL, et al. Safety and efficacy of intratumoral injections of chimeric antigen receptor (CAR) T cells in metastatic breast cancer[J]. Cancer Immunol Res, 2017, 5(12):1152-1161. doi: 10.1158/2326-6066.CIR-17-0189 [18] Migliorini D, Dietrich PY, Stupp R, et al. CAR T-Cell therapies in glioblastoma: a first look[J]. Clin Cancer Res, 2018, 24(3):535. doi: 10.1158/1078-0432.CCR-17-2871 [19] Newick K, O'Brien S, Moon E, et al. CAR T cell therapy for solid tumors[J]. Annu Rev Med, 2017, (68):139-152. http://www.ncbi.nlm.nih.gov/pubmed/27860544 [20] Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion[J]. Sci, 2011, 331(6024):1565-1570. doi: 10.1126/science.1203486 [21] Dunn GP, Old LJ, Schreiber RD. The Immunobiology of cancer immunosurveillance and immunoediting[J]. Immunity, 2004, 21(2):137-148. doi: 10.1016/j.immuni.2004.07.017 [22] Ok CY, Yong KH. Checkpoint inhibitors in hematological malignancies[J]. J Hematol Oncol, 2017, 10(1):103. doi: 10.1186/s13045-017-0474-3 [23] Zhu X, Lang J. Programmed death-1 pathway blockade produces a synergistic antitumor effect: combined application in ovarian cancer[J]. J Gynecol Oncol, 2017, 28(5):e64. doi: 10.3802/jgo.2017.28.e64 [24] Ribas A, Shin DS, Zaretsky J, et al. PD-1 blockade expands intratumoral memory T cells[J]. Cancer Immunol Res, 2016, 4(3):194-203. doi: 10.1158/2326-6066.CIR-15-0210 [25] Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 Blockade[J]. Sci, 1996, 271(5256):1734-1736. doi: 10.1126/science.271.5256.1734 [26] Ren Z, Guo J, Liao J, et al. CTLA-4 limits anti-CD20-mediated tumor regression[J]. Clin Cancer Res, 2017, 23(1):193-203. doi: 10.1158/1078-0432.CCR-16-0040 [27] Merchant MS, Wright M, Baird K, et al. PhaseⅠclinical trial of ipilimumab in pediatric patients with advanced solid tumors[J]. Clin Cancer Res, 2016, 22(6):1364-1370. doi: 10.1158/1078-0432.CCR-15-0491 [28] Sakamuri D, Glitza IC, Betancourt Cuellar SL, et al. PhaseⅠdose-escalation study of anti-CTLA-4 antibody ipilimumab and lenalidomide in patients with advanced cancers[J]. Mol Cancer Thera, 2018, 17(3): 671. doi: 10.1158/1535-7163.MCT-17-0673 [29] Zhou G, Sprengers D, Boor PPC, et al. Antibodies against immune checkpoint molecules restore functions of tumor-infiltrating T cells in hepatocellular carcinomas[J]. Gastroenter, 2017, 153(4):1107-1119. doi: 10.1053/j.gastro.2017.06.017 [30] Yang ZZ, Price-troska T, Novak AJ, et al. The exhausted intratumoral T cell population in B-cell non-hodgkin lymphoma is defined by LAG-3, PD-1 andtim-3 expression[J]. Blood, 2015, (126):2661-2661. http://www.bloodjournal.org/content/126/23/2661 [31] Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation [J]. Cell, 2011, 144(5):646-674. doi: 10.1016/j.cell.2011.02.013 [32] Rosenblatt J, Avigan D. Targeting the PD-1/PD-L1 axis in multiple myeloma: a dream or a reality[J]? Blood, 2017, 129(3):275-279. doi: 10.1182/blood-2016-08-731885 [33] Yeku OO, Purdon TJ, Koneru M, et al. Armored CAR-T cells enhance antitumor efficacy and overcome the tumor microenvironment[J]. Sci Rep, 2017, 7(1):10541. doi: 10.1038/s41598-017-10940-8 [34] Gargett T, Yu W, Dotti G, et al. GD2-specific CAR-T cells undergo potent activation and deletion following antigen encounter but can be protected from activation-induced cell death by PD-1 blockade [J]. Mol Ther, 2016, 24(6):1135-1149. doi: 10.1038/mt.2016.63 [35] Li S, Siriwon N, Zhang X, et al. Enhanced cancer immunotherapy by chimeric antigen receptor–modified T cells engineered to secrete checkpoint inhibitors[J]. Clin Cancer Res, 2017, 23(22):6982-6992. doi: 10.1158/1078-0432.CCR-17-0867 [36] Chong EA, Melenhorst JJ, Lacey SF, et al. PD-1 blockade modulates chimeric antigen receptor (CAR)-modified T cells: refueling the CAR [J]. Blood, 2017, 129(8):1039-1041. doi: 10.1182/blood-2016-09-738245 [37] Chong EA, Melenhorst JJ, Svoboda J, et al. PhaseⅠ/Ⅱstudy of pembrolizumab for progressive diffuse large B cell lymphoma after antiCD19 directed chimeric antigen receptor modified T cell therapy[J]. Blood, 2017, 130(Suppl 1):4121-4121. [38] Liu X, Ranganathan R, Jiang S, et al. A chimeric switch-receptor targeting PD1 augments the efficacy of second-generation CAR-T cells in advanced solid tumors[J]. Cancer Res, 2016, 76(6):1578-1590. doi: 10.1158/0008-5472.CAN-15-2524 [39] Heczey A, Louis CU, Savoldo B, et al. CAR-T cells administered in combination with lymphodepletion and PD-1 inhibition to patients with neuroblastoma[J]. Mol Ther, 2017, 25(9):2214-2224. doi: 10.1016/j.ymthe.2017.05.012 [40] Condomines M, Arnason J, Benjamin R, et al. Tumor-targeted human T cells expressing CD28-based chimeric antigen receptors circumvent CTLA-4 inhibition[J]. PLoS One, 2015, 10(6):e0130518. doi: 10.1371/journal.pone.0130518 [41] Schuster SJ, Svoboda J, Chong EA, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas[J]. New Engl J Med, 2017, 377 (26):2545-2554. doi: 10.1056/NEJMoa1708566 [42] Kenderian SS, Ruella M, Shestova O, et al. Identification of PD1 and TIM3 as checkpoints that limit chimeric antigen receptor T cell efficacy in leukemia[J]. Blood, 2015, 126(23):852-852. http://www.sciencedirect.com/science/article/pii/S1083879115010484 [43] Beavis PA, Henderson MA, Giuffrida L, et al. Targeting the adenosine 2A receptor enhances chimeric antigen receptor T cell efficacy [J]. J Clin Invest, 2017, 127(3):929-941. doi: 10.1172/JCI89455 -




 下载:
下载: