Safety analysis of implanted peritoneal ports in gastric cancer with peritoneal metastasis
-
摘要:
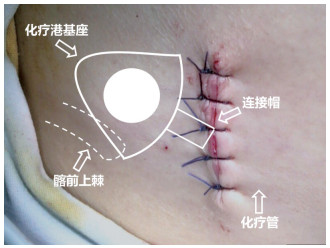
目的 腹腔化疗可将药物直接作用于腹膜转移病灶,正逐渐被应用于胃癌腹膜转移的治疗。通过腹腔化疗港可反复多次进行腹腔化疗,而化疗港的顺利使用是腹腔化疗持续进行的关键。本研究将探讨在胃癌腹膜转移患者留置腹腔化疗港的安全性。 方法 回顾性分析2015年6月至2018年6月于北京大学肿瘤医院行腹腔化疗港留置术患者的临床资料,分析腹腔化疗港在胃癌腹膜转移中应用的过程、注意事项及安全性,探讨并发症发生原因及预防处理方式。 结果 共有54例患者植入55个化疗港,平均使用时间为8.4(0.8~32.0)个月,共13例出现并发症(23.6%),发生率从高到低依次为堵塞(7.3%)、严重疼痛(5.5%)、感染(3.6%)、反流(3.6%)、置针困难(1.8%)及皮下硬结(1.8%)。并发症发生的中位时间为化疗港放置后2.1个月。分析未发现与并发症相关的高危因素(P > 0.05)。 结论 通过规范与细致的操作,腹腔化疗港在胃癌腹膜转移中的应用安全可行。 Abstract:Objective Intraperitoneal chemotherapy is increasingly being used in the treatment of gastric cancer with peritoneal metastasis, because the drug can directly act on the metastatic nodules. Repeated treatment can be administered through implanted ports, provided the ports are appropriately managed. Our study aimed to investigate the safety of peritoneal port implantation in patients with gastric cancer with peritoneal metastasis. Methods We retrospectively reviewed the records of patients undergoing intraperitoneal port implantation for the administration of chemotherapy between June 2015 and June 2018 to investigate the causes of complications and to discuss their management and prevention. Results Fifty-five ports were implanted in 54 patients with median usage time of 8.4 (0.8-32.0) months. Complications occurred at 13 port sites (23.6%), including obstruction (7.3%), severe pain (5.5%), infection (3.6%), reflux (3.6%), access difficulty (1.8%), and subcutaneous mass formation (1.8%). The median interval from the time of port implantation to the development of complications was 2.1 months. No factor contributing to the complications was identified (P> 0.05). Conclusions Peritoneal port implantation to systematic chemotherapy in patients with gastric cancer with peritoneal metastasis is safe and feasible if the ports can be carefully managed. -
Key words:
- gastric cancer /
- peritoneal metastasis /
- implanted peritoneal ports /
- complication /
- safety
-
表 1 留置腹腔化疗港患者的临床病理特点

表 2 化疗港相关并发症

表 3 腹腔化疗港并发症相关因素

-
[1] Chen W, Zheng R, Zhang S, et al. Cancer incidence and mortality in China in 2013: an analysis based on urbanization level[J]. Chin J Cancer Res, 2017, 29(1):1-10. doi: 10.21147/j.issn.1000-9604.2017.01.01 [2] Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase Ⅲ trial[J]. Lancet Oncol, 2008, 9(3):215-221. doi: 10.1016/S1470-2045(08)70035-4 [3] Ajani JA, Rodriguez W, Bodoky G, et al. Multicenter phase Ⅲ comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial[J]. J Clin Oncol, 2010, 28(9):1547-1553. doi: 10.1200/JCO.2009.25.4706 [4] Jacquet P, Sugarbaker PH. Peritoneal-plasma barrier[J]. Cancer Treat Res, 1996, 82:53-63. doi: 10.1007/978-1-4613-1247-5 [5] Hasovits C, Clarke S. Pharmacokinetics and pharmacodynamics of intraperitoneal cancer chemotherapeutics[J]. Clin Pharmacokinet, 2012, 51(4):203-224. doi: 10.2165/11598890-000000000-00000 [6] 薛侃, 李子禹.浅析腹腔化疗港在胃癌腹膜转移治疗中的应用[J].国际外科学杂志, 2018, 45(4):217-220. doi: 10.3760/cma.j.issn.1673-4203.2018.04.001 [7] Ishigami H, Fujiwara Y, Fukushima R, et al. Phase Ⅲ trial comparing intraperitoneal and intravenous paclitaxel plus S-1 versus cisplatin plus s- 1 in patients with gastric cancer with peritoneal metastasis: PHOENIX-GC trial[J]. J Clin Oncol, 2018, 36(19):1922-1929. doi: 10.1200/JCO.2018.77.8613 [8] Ishigami H, Yamaguchi H, Yamashita H, et al. Surgery after intraperitoneal and systemic chemotherapy for gastric cancer with peritoneal metastasis or positive peritoneal cytology findings[J]. Gastric Cancer, 2017, 20(Suppl 1):128-134. http://d.old.wanfangdata.com.cn/Periodical/wjg200546027 [9] 薛侃, 李子禹, 李双喜, 等.腹腔镜热灌注化疗联合腹腔及系统化疗转化治疗胃癌腹膜转移病人1例报告[J].中国实用外科杂志, 2017, 37 (10):67-70. http://www.cqvip.com/QK/90965A/201710/673574045.html [10] Walker JL, Armstrong DK, Huang HQ, et al. Intraperitoneal catheter outcomes in a phase Ⅲ trial of intravenous versus intraperitoneal chemotherapy in optimal stage Ⅲ ovarian and primary peritoneal cancer: a gynecologic oncology group study[J]. Gynecol Oncol, 2006, 100(1):27-32. http://en.cnki.com.cn/Article_en/CJFDTotal-SJFC2006Z1076.htm [11] Topuz E, Saip P, Aydiner A, et al. Catheter complications associated with intraperitoneal chemotherapy[J]. Eur J Gynaecol Oncol, 1998, 19(3): 275-279. doi: 10.1016-0090-8258(86)90006-5/ [12] Topuz E, Salihoglu Y, Aydiner A, et al. Celsite port and catheter as an intraperitoneal access device in the treatment of ovarian cancer[J]. J Surg Oncol, 2000, 74(3):223-226. doi: 10.1002/(ISSN)1096-9098 [13] Li Z, Li Z, Jia S, et al. Depth of tumor invasion and tumor-occupied portions of stomach are predictive factors of intra- abdominal metastasis[J]. Chin J Cancer Res, 2017, 29(2):109-117. doi: 10.21147/j.issn.1000-9604.2017.02.03 [14] Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis[J]. Cancer Treat Res, 1996, 82: 359-374. doi: 10.1007/978-1-4613-1247-5 [15] Emoto S, Ishigami H, Hidemura A, et al. Complications and management of an implanted intraperitoneal access port system for intraperitoneal chemotherapy for gastric cancer with peritoneal metastasis [J]. Jpn J Clin Oncol, 2012, 42(11):1013-1019. doi: 10.1093/jjco/hys129 [16] Alberts DS, Liu PY, Hannigan EV, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage Ⅲ ovarian cancer[J]. N Engl J Med, 1996, 335(26):1950-1955. doi: 10.1056/NEJM199612263352603 [17] Helm CW. Ports and complications for intraperitoneal chemotherapy delivery[J]. BJOG, 2012, 119(2):150-159. doi: 10.1111/j.1471-0528.2011.03179.x [18] Lesnock JL, Richard SD, Zorn KK, et al. Completion of intraperitoneal chemotherapy in advanced ovarian cancer and catheter- related complications[J]. Gynecol Oncol, 2010, 116(3):345-350. doi: 10.1016/j.ygyno.2009.11.009 [19] Almadrones L, Yerys C. Problems associated with the administration of intraperitoneal therapy using the Port-A-Cath system[J]. Oncol Nurs Forum, 1990, 17(1):75-80. http://www.ncbi.nlm.nih.gov/pubmed/2300507 [20] Roveron G, De Toma G, Barbierato M. Italian society of surgery and association of stoma care nurses joint position statement on preoperative stoma siting[J]. J Wound Ostomy Continence Nurs, 2016, 43(2): 165-169. doi: 10.1097/WON.0000000000000204 [21] Makhija S, Leitao M, Sabbatini P, et al. Complications associated with intraperitoneal chemotherapy catheters[J]. Gynecol Oncol, 2001, 81 (1):77-81. http://d.old.wanfangdata.com.cn/OAPaper/oai_doaj-articles_3b03394d3e9bdd6b40e9b0891ec9e51e [22] Malmström H, Carstensen J, Simonsen E. Experience with implanted subcutaneous ports for intraperitoneal chemotherapy in ovarian cancer[J]. Gynecol Oncol, 1994, 54(1):27-34. doi: 10.1006-gyno.1994.1161/ [23] Landrum LM, Gold MA, Moore KN, et al. Intraperitoneal chemotherapy for patients with advanced epithelial ovarian cancer: a review of complications and completion rates[J]. Gynecol Oncol, 2008, 108(2): 342-347. http://cat.inist.fr/?aModele=afficheN&cpsidt=20087741 [24] Robinson WR, Beyer J. Factors affecting the completion of intraperitoneal chemotherapy in women with ovarian cancer[J]. Int J Gynecol Cancer, 2010, 20(1):70-74. doi: 10.1111/IGC.0b013e3181c7f670 -




 下载:
下载: