Eleven cases of DEB-TACE comprehensive interventional strategy for stage Ⅲa hypovascular hepatocellular carcinoma
-
摘要:
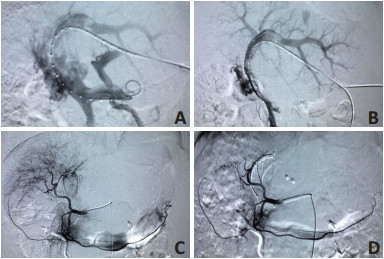
目的 探讨门静脉支架联合125I粒子条植入、载药微球经肝动脉化疗栓塞术(drug-eluting beads transarterial chemoembolization,DEB-TACE)及分子靶向药物综合治疗Ⅲa期乏血供肝细胞癌(hepatocellular carcinoma,HCC)伴门静脉癌栓(portal vein tumor thrombus,PVTT)的安全性和有效性。 方法 回顾性分析2016年11月至2018年10月于华中科持大学同济医学院附属协和医院11例确诊为Ⅲa期乏血供HCC伴PVTT 11例患者,行门静脉支架联合125I粒子条植入后序贯使用载药微球经肝动脉化学栓塞术(DEB-TACE)及分子靶向药物综合治疗。随访期间,评估所有患者治疗后支架通畅情况及DEB-TACE治疗后的肿瘤反应,比较术前、术后1个月的肝功能、血常规的变化并总结并发症的发生情况。 结果 根据原发性肝癌诊疗规范(2017年版)11例患者均为Ⅲa期,Child-Pugh A、B级,影像学提示为乏血供肝癌,最大径为8.4±4.1(2.8~14.1)cm,均伴有PVTT,其中程氏分型Ⅱ型者4例,Ⅲ型者7例;门静脉主干癌栓≥50%者6例, < 50%者1例。所有患者顺利进行125I粒子支架门静脉内植入联合DEB-TACE治疗。支架植入后3个月、6个月通畅率均为100%;DEB-TACE治疗后3个月完全缓解(complete response,CR)患者4例(36.4%),部分缓解(partial response,PR)患者5例(45.5%),疾病稳定(stable disease,SD)患者2例(18.2%),PD患者0例。客观反应率(objective response rate,ORR)为81.8%,疾病控制率(disease control rate,DCR)为100%。肝肾功能、血常规等指标术前与术后1个月差异无统计学意义,11例患者在围手术期过程中未出现严重并发症。 结论 门静脉支架联合125I粒子条植入序贯使用载药微球经肝动脉化学栓塞术(DEB-TACE)及分子靶向药物,综合治疗Ⅲa期乏血供HCC伴PVTT可以恢复门脉主干血流并保持中长期通畅,同时较理想地杀灭肿瘤及控制肿瘤生长,是安全有效的治疗策略。 Abstract:Objective To evaluate the efficacy and safety of portal vein stenting combined with 125I particle strand implantation followed by drug-eluting beads transarterial chemoembolization (DEB-TACE) and molecular-targeted therapy for the treatment of stage Ⅲa liver cancer lacking a blood supply. Methods A retrospective analysis of 11 patients who had stage Ⅲa liver cancer lacking a blood supply combined with portal vein tumor thrombosis (PVTT) was conducted from October 2016 to October 2018. All the patients underwent portal vein stenting combined with 125I particle strand implantation, DEB-TACE, and comprehensive treatment containing molecular-targeted drugs. During the follow-up period, all patients were evaluated for stent patency after the implantation and tumor response after DEB-TACE treatment. The liver function and blood routine changes before and 1 month after the surgery were completed, and the complications were summarized. Results All 11 patients were judged as stage Ⅲa liver cancer based on the Chinese staging criteria (2017), Child-Pugh classification grade A and B. The imaging findings indicated that these tumors were hypovascular. The maximum diameter of these lesions was (8.4±4.1) (2.8-14.1) cm, and all patients had PVTT. Among them, there were 4 cases of Cheng's type Ⅱ and 7 cases of type Ⅲ: 6 cases of main PVTT ≥50% and 1 case of PVTT < 50%. All patients underwent portal vein stenting combined with 125I particle strand implantation, DEB-TACE, and comprehensive treatment containing molecular-targeted drugs. Three and 6 months after stent implantation, the patency rate was 100%; 3 months after DEB-TACE treatment, complete response was achieved in 4 (36.4%) patients, partial response was achieved in 5 (45.5%) patients, and stable disease was achieved in 2 (18.2%) patients. No patients exhibited progressive disease. Therefore, the objective response rate was 81.8% and disease control rate was 100%. As for the liver and kidney function and blood routine tests, there were no significant differences between baseline and 1 month after the surgery. In addition, no patient had any serious complication during the perioperative period. Conclusions For patients with stage Ⅲa liver cancer lacking a blood supply and PVTT, a comprehensive treatment strategy including portal vein stenting combined with 125I particle strand implantation, DEB-TACE, and molecular-targeted therapy can restore portal vein blood flow and maintain mid- and longterm stent patency, while effectively killing tumors and controlling tumor growth, which is a safe and effective treatment strategy. -
Key words:
- hepatocellular carcinoma (HCC) /
- portal vein stent /
- 125I seed /
- drug-eluting beads
-
表 1 患者基线特征

表 2 患者术前及术后1个月血常规及肝功能比较

-
[1] Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6):394-424. http://cn.bing.com/academic/profile?id=72ddb2c2747f7f6244ee8ffc4ce98b19&encoded=0&v=paper_preview&mkt=zh-cn [2] Zhang ZM, Lai EC, Zhang C, et al. The strategies for treating primary hepatocellular carcinoma with portal vein tumor thrombus[J]. Int J Surg, 2015, 20:8-16. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=fbwk201405020 [3] Katagiri S, Yamamoto M. Multidisciplinary treatments for hepatocellular carcinoma with major portal vein tumor thrombus[J]. Sur Today, 2014, 44(2):219-226. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=969c2749a4b6bc05188930edda8ab90e [4] Zhang ZH, Liu QX, Zhang W, et al.Combined endovascular brachytherapy, sorafenib, and transarterial chemobolization therapy for hepatocellular carcinoma patients with portal vein tumor thrombus[J]. World J Gastroenterol, 2017, 23(43):7735-7745. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QKC20172017121900134890 [5] Cao G, Zhu X, Li J, et al.A comparative study between Embosphere((R)) and conventional transcatheter arterial chemoembolization for treatment of unresectable liver metastasis from GIST[J]. Chin J Cancer Res, 2014, 26(1):124-131. http://cn.bing.com/academic/profile?id=13e5275d3c4c6d28d1b4cc7b130b3bdb&encoded=0&v=paper_preview&mkt=zh-cn [6] 樊嘉, 秦叔逵, 沈锋, 等.原发性肝癌诊疗规范(2017年版)[J].中国实用外科杂志, 2017(7):705-720. http://d.old.wanfangdata.com.cn/Periodical/crbxx201703001 [7] 程树群, 吴孟超, 陈汉, 等.肝癌门静脉癌栓分型的影像学意义[J].中华普通外科杂志, 2004, (4):6-7. http://d.old.wanfangdata.com.cn/Periodical/zhptwk200404002 [8] Lencioni R, Llovet JM. Modified RECIST (mRECIST) Assessment for Hepatocellular Carcinoma[J]. Semin Liver Dis, 2010, 30(1):52-60. doi: 10.1055/s-0030-1247132 [9] 吴孟超, 程树群, 陈敏超, 等.肝细胞肝癌合并门静脉癌栓多学科诊治中国共识(2016年版)(讨论稿)[J].肝癌电子杂志, 2016(1):1-14. http://d.old.wanfangdata.com.cn/Periodical/gadzzz201601002 [10] Lu J, Guo JH, Zhu HD, et al. Safety and efficacy of irradiation stent placement for malignant portal vein thrombus combined with transarterial chemoembolization for hepatocellular carcinoma: a singlecenter experience[J]. J Vasc Interv Radiol, 2017, 28(6):786-794. doi: 10.1016/j.jvir.2017.02.014 [11] Yu TZ, Zhang W, Liu QX, et al. Endovascular brachytherapy combined with portal vein stenting and transarterial chemoembolization improves overall survival of hepatocellular carcinoma patients with main portal vein tumor thrombus[J]. Oncotarget, 2017, 8(7):12108. http://cn.bing.com/academic/profile?id=c4043cc74941fc4cd26e796d43977f11&encoded=0&v=paper_preview&mkt=zh-cn [12] Liu YS, Lin CY, Chuang MT, et al. Five-year outcome of conventional and drug-eluting transcatheter arterial chemoembolization in patients with hepatocellular carcinoma[J]. BMC Gastroenterology, 2018, 18(1). http://cn.bing.com/academic/profile?id=1a2c91966c2f4663aaed9053c0d397bc&encoded=0&v=paper_preview&mkt=zh-cn [13] Lammer J, Malagari K, Vogl T, et al. Prospective Randomized Study of Doxorubicin-Eluting-Bead Embolization in the Treatment of Hepatocellular Carcinoma: Results of the PRECISION V Study[J]. CardioVascular and Interventional Radiology, 2010. 33(1):41-52. doi: 10.1007/s00270-009-9711-7 [14] Martin RC, Salem R, Adam R, et al. Locoregional surgical and interventional therapies for advanced colorectal cancer liver metastases: expert consensus statements[J]. HPB(Oxford), 2013, 15(2):131-133. http://cn.bing.com/academic/profile?id=1332cd13f052327c9ca0193f3c5f6fd3&encoded=0&v=paper_preview&mkt=zh-cn [15] Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma[J]. New Engl J Med, 2008, 359(4):378-390. doi: 10.1056/NEJMoa0708857 [16] Jeong SW, Jang JY, Shim KY, et al. Practical Effect of Sorafenib Monotherapy on Advanced Hepatocellular Carcinoma and Portal Vein Tumor Thrombosis[J]. Gut Liver, 2013, 7(6):696-703. doi: 10.5009/gnl.2013.7.6.696 -




 下载:
下载: