Expression of CD30 and its correlation with prognosis of patients with peripheral T cell lymphoma, unspecified
-
摘要:
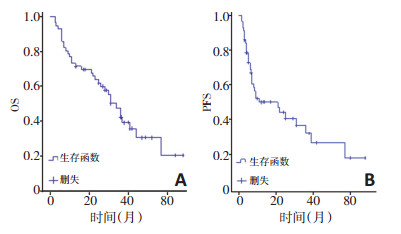
目的 分析CD30在外周T细胞淋巴瘤-非特指型(peripheral lymphoma T cell lymphoma, unspecified, PTCL-U)中的表达情况,研究CD30与临床生存预后的相关性。 方法 收集2013年1月至2017年12月郑州大学第一附属医院初治的56例PTCL-U患者的病例资料,男女比例为1.7:1,中位年龄60(16~76)岁。分类变量行Chi-square检验,采用Kaplan-Meier方法、Logistic单因素分析及Cox回归模型多因素分析进行生存资料分析。 结果 56例患者3、5年总生存(overall survival, OS)率分别为42.2%和20.4%, 无进展生存(progression free survival, PFS)率分别为32.1%和17.8%, 中位总生存期(median OS, mOS)为31个月, 中位无进展生存期(median PFS, mPFS)为11个月。CD30在PTCL-U中的阳性表达率为35.7%, CD30阳性表达在晚期患者、LDH水平升高、结外病变数目≥2个部位、中-高危患者中更为多见, 阳性患者初治效果不佳(P < 0.05)。56例患者化疗采用CHOP与GDPT方案, 两种方案对OS及PFS的影响差异无统计学意义(P>0.05)。CD30阳性组与阴性组的3年OS分别为16.5%与54.9%(P=0.001); 3年PFS分别为11.2%与44.5%(P=0.016)。单因素分析显示CD30阳性晚期患者、CD30阳性、IPI/aaIPI评分中-高危及PIT中-高危为预后不利因素, 多因素分析显示分期、PIT评分与生存期相关。 结论 CD30在PTCL-U中的表达率偏低,阳性表达组生存预后差;CD30阳性在晚期患者、LDH水平升高、中-高危组表达更多,阳性组更易累及结外、客观缓解率较低;分期、CD30、IPI/aaIPI评分和PIT影响患者的预后。 Abstract:Objective The expression of CD30 in peripheral lymphoma T cell lymphoma, unspecified (PTCL-U) was analyzed, and the correlations between CD30 and clinical survival and prognosis were studied. Methods The clinical and pathological indicators of 56 patients with PTCL-U, who were newly treated in The First Affiliated Hospital of Zhengzhou University from January 2013 to December 2017, were obtained. Among the 56 patients, the male to female ratio was 1.7:1. The median age was 60 (16-76) years. The categorical variables were analyzed by the Chi-square test. Kaplan-Meier method was used for the survival analysis along with an assessment of the differences by Log-rank test. Logistic univariate analysis and Cox multivariate regression model analysis were used to analyze the indicators affecting survival. Results The 3-year and 5-year overall survival (OS) rates of 56 patients were 42.2% and 20.4%, respectively, and the progression-free survival (PFS) rates were 32.1% and 17.8%, respectively. The median overall survival (median-OS, mOS) was 31 months, and the median progressionfree survival (median-PFS, mPFS) was 11 months. The positive expression rate of CD30 in PTCL-U patients was 35.7%. The positive expression of CD30 was more common in advanced patients. The LDH level was increased, and the number of extra-nodal lesions was ≥2 in the middlehigh risk patients. Further, the initial treatment effects in the positive patients were not good (P < 0.05). Among the 56 patients, CHOP regimen and GDPT regimen were adopted for chemotherapy, and the two regimens showed no statistically significant effects on OS and PFS (P>0.05). The survivals in the CD30 positive and negative groups were as follows: the 3 years-OS were 16.5% and 54.9%, respectively (P=0.001); and the 3-years PFS were 11.2% and 44.5%, respectively (P=0.016). The univariate analysis showed that advanced disease, CD30-positivity, IPI/ aaIPI-high risk, and PIT-high risk (P>0.05) were adverse prognostic factors. The multivariate analysis showed that staging and PIT were correlated with survival. Conclusions The expression of CD30 in PTCL-U was low, and the prognosis of the positive-expression group was poor. The positive expression was more associated with advanced disease, high level of LDH, and medium-high risk group. The positive group was more prone to extranodal involvement and the objective remission rate was lower. Staging, CD30, IPI/aaIPI, and PIT could affect the prognosis in these patients. -
表 1 CD30表达与临床特点、病理学指标及初治效果的关系

表 2 PTCL-U患者的单因素分析

表 3 PTCL-U患者的多因素分析

-
[1] Broccoli A, Zinzani PL. Peripheral T-cell lymphoma, not otherwise specified[J]. J Family Med Prim Care, 2017, 6(2):427-430. [2] Alexander S, Misono B, Ad S, et al. Peripheral T-cell lymphoma, not otherwise specified: An unusual presentation of a rare lymphoma [J]. Cureus, 2019, 11(1):e3813. http://cn.bing.com/academic/profile?id=bb2c2e8b54fc866fd3e23f4f934b3824&encoded=0&v=paper_preview&mkt=zh-cn [3] Ranuhardy D, Suzanna E, Sari RM, et al. CD30, CD15, CD50, and PAX5 expressions as diagnostic markers for Hodgkin lymphoma (HL) and systemic anaplastic large cell lymphoma (sALCL)[J]. Acta Medica Indonesiana, 2018, 50(2):104-109. http://cn.bing.com/academic/profile?id=cb0aaa9bae94c0ddbd20dd8eab6940c9&encoded=0&v=paper_preview&mkt=zh-cn [4] Steven H, Oa O, Pro B, et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial[J]. Lancet (London, England), 2019, 393(1068):229-240. http://cn.bing.com/academic/profile?id=330c26eac6a6df1732c0a0173dbf321d&encoded=0&v=paper_preview&mkt=zh-cn [5] Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification[J]. J Clin Oncol, 2014, 32(27):3059-3068. doi: 10.1200/JCO.2013.54.8800 [6] Yasuo A, Masaru T, Naoki W, et al. Gemcitabine, cisplatin, and dexamethasone (GDP) in combination with methotrexate and pegaspargase is active in newly diagnosed peripheral T cell lymphoma patients: a phase 2, single-center, open-label study in China[J]. Ann Hematol, 2019, 98(1):143-150. doi: 10.1007/s00277-018-3488-1 [7] Xu P, Yu D, Wang L, et al. Analysis of prognostic factors and comparison of prognostic scores in peripheral T cell lymphoma, not otherwise specified: a single-institution study of 105 Chinese patients[J]. Ann Hematol, 2015, 94(2):239-247. doi: 10.1007/s00277-014-2188-8 [8] Ai-zahrani M, Savage KJ. Peripheral T-cell lymphoma, not otherwise specified[J]. Hematol/Oncol Clin of North Am, 2017, 31(2):189-207. doi: 10.1016/j.hoc.2016.11.009 [9] Aneja A, LevakaVeera R, Patel V, et al. Aggressive subcutaneous panniculitis-like CD30+ peripheral T-Cell lymphoma with diffuse EBER expression[J]. Case Report Hematol, 2014, 2014:1-4. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=Doaj000004486103 [10] Nakashima M, Watanabe M, Uchimaru K, et al. Trogocytosis of ligand-receptor complex and its intracellular transport in CD30 signalling[J]. Biol Cell, 2018, 110(5):109-124. http://cn.bing.com/academic/profile?id=7609e2bae905bea5cdea371190cf3527&encoded=0&v=paper_preview&mkt=zh-cn [11] Louza N, Jinguely Z, St R, et al. CD30-positive lymphoproliferative disorders[J]. Cancer Treat Res, 2019, 51(6):249-268. http://d.old.wanfangdata.com.cn/Periodical/zhpf201103001 [12] Wj L, Ij M, Hj S, et al. CD30-positive cutaneous extranodal natural killer/T-cell lymphoma: clinicopathological features and survival outcomes[J]. Int J Dermatol, 2019, 58(6):688-696. doi: 10.1111/ijd.2019.58.issue-6 [13] Makoto N, Tadanori Y, Mariko W, et al. CD30 characterizes polylobated lymphocytes and disease progression in HTLV-1-infected individuals[J]. Clin Cancer Res, 2018, 24(21):5445-5457. http://cn.bing.com/academic/profile?id=49578364c93023003229928d361144c7&encoded=0&v=paper_preview&mkt=zh-cn [14] Céline B, Mp D, Marie P, et al. Immunohistochemistry as a valuable tool to assess CD30 expression in peripheral T-cell lymphomas: high correlation with mRNA levels[J]. Blood, 2014, 124(19):2983-2986. doi: 10.1182/blood-2014-07-584953 [15] Weisenburger DD, Savage KJ, Nancy LH, et al. Peripheral T-cell lymphoma, not otherwise specified: a report of 340 cases from the international peripheral T-cell lymphoma project[J]. Blood, 2011, 117 (12):3402-3408. doi: 10.1182/blood-2010-09-310342 [16] Bisig B, de Reyniès A, Bonnet C, et al. CD30-positive peripheral Tcell lymphomas share molecular and phenotypic features[J]. Haematologica, 2013, 98(8):1250-1258. doi: 10.3324/haematol.2012.081935 [17] Sm H, Rh A, Nl B, et al. Objective responses in relapsed T-cell lymphomas with single-agent brentuximab vedotin[J]. Blood, 2014, 123(20):3095-3100. doi: 10.1182/blood-2013-12-542142 -




 下载:
下载: