-
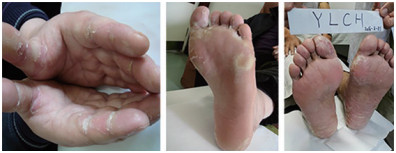
摘要: 甲磺酸阿帕替尼片为一种新型小分子单克隆抗体,作用机制是高度选择性竞争细胞内血管内皮生长因子受体-2(vascularendothelial growth factor receptor-2,VEGFR-2)的ATP结合位点,阻断下游信号转导,抑制肿瘤组织新血管生成。手足皮肤反应(hand-foot skin reaction,HFSR)为阿帕替尼常见的不良反应之一,影响患者的生存质量,导致靶向药减量,从而影响抗肿瘤治疗的疗效。常规的西医治疗为尿素和糖皮质激素外用制剂,临床常根据红肿、破溃、结痂等不同皮肤表现的进行中西医结合治疗。本文对阿帕替尼相关HFSR的表现及治疗进展予以综述,以期给临床提供参考。Abstract: Apatinib mesylate is a novel small molecule monoclonal antibody. Its mechanism of action is through highly selective competition for intracellular vascular endothelial growth factor receptor-2 (VEGFR-2) ATP binding sites and blocking downstream signal transduction thereby inhibiting neovascularization of tumor tissue. Hand-foot skin reaction (HFSR) is one of the common adverse reactions of apatinib, and it often affects the quality of life of patients; this leads to a reduction in the use of targeted drugs, which negatively affects the efficacy of anti-tumor therapy. The conventional western medicine treatment for HFSR comprises urea and glucocorticoid topical preparations. Clinical treatment is often based on redness, ulceration, scab, and other skin manifestations; a combination of Chinese and western medicines can improve clinical outcomes. This article reviews apatinib-related HFSR and the relevant progress in treatment strategies in order to provide a clinical reference.
-
Key words:
- apatinib /
- hand-foot skin reaction (HFSR) /
- clinical manifestation /
- therapy /
- study advances
-
表 1 阿帕替尼在临床试验中HFSR的发生率

-
[1] Li J, Qin Sk, Xu JM, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer:results from a randomized, placebo-controlled, parallel-arm, phaseⅡtrial[J]. J Clin Oncol, 2013, 31(26):3219-3225. doi: 10.1200/JCO.2013.48.8585 [2] Degen A, Alter M, Schenck F, et al. The hand-foot-syndrome associated with medical tumor therapy-classification and management[J]. J Dtsch Dermatol Ges, 2010, 8(9):652-661. http://cn.bing.com/academic/profile?id=74178a97d933f87989a70e3517a15e96&encoded=0&v=paper_preview&mkt=zh-cn [3] Baack BR, Burgdorf WH. Chemotherapy induced acral erythema[J]. J Am Acad Dermatol, 1991, 24(3):457-461. doi: 10.1016/0190-9622(91)70073-B [4] Nagore E, Insa A, Sanmartín O. Antineoplastic therapy-induced palmar plantar erythrodysesthesia ("hand-foot") syndrome:incidence, recognition and management[J]. Am J Clin Dermatol, 2000, 1(4):225-234. doi: 10.2165/00128071-200001040-00004 [5] National Cancer Institute. Common terminology criteria for adverse events(CTCAE) version 4.0. http://evs.nci.nih.gov/ftp1/CTCAE/About.html. (1 September 2017, date last accessed). [6] Gou M, Si H, Zhang Y, et al. Efficacy and safety of apatinib in patients with previously treated metastatic colorectal cancer:a realworld retrospective study[J]. Sci Rep, 2018, 8(1):4602. [7] Miao M, Deng G, Luo S, et al. A phaseⅡstudy of apatinib in patients with recurrent epithelial ovarian cancer[J]. Gynecol Oncol, 2018, 148(2):286-290. doi: 10.1016/j.ygyno.2017.12.013 [8] Ruan H, Dong J, Zhou X, et al. Multicenter phase Ⅱ study of apatinib treatment for metastatic gastric cancer after failure of secondline chemotherapy[J]. Oncotarget, 2017, 8(61):104552-104559. [9] Zhang Y, Han C, Li J, et al. Efficacy and safety for Apatinib treatment in advanced gastric cancer:a real world study[J]. Sci Rep, 2017, 7(1):13208. doi: 10.1038/s41598-017-13192-8 [10] Zeng DX, Wang CG, Lei W, et al. Efficiency of low dosage apatinib in post-first-line treatment of advanced lung adenocarcinoma[J]. Oncotarget, 2017, 8(39):66248-66253. http://cn.bing.com/academic/profile?id=f20191931a88dd649d642256b0223f87&encoded=0&v=paper_preview&mkt=zh-cn [11] 姚艺玮, 何义富, 胡冰, 等.阿帕替尼治疗晚期胃癌临床观察[J].中华肿瘤防治杂志, 2017, 24(6):389-393. http://www.cnki.com.cn/Article/CJFDTotal-QLZL201706007.htm [12] 郎丰平, 赵毓毅, 范鹏.甲磺酸阿帕替尼治疗晚期胃癌的疗效及安全性分析[J].实用癌症杂志, 2017, 32(6):996-998. doi: 10.3969/j.issn.1001-5930.2017.06.037 [13] Cho YT, Chan CC. Cabozantinib-induced hand-foot skin reaction with subungual splinter hemorrhages and hypertension:a possible association with inhibition of the vascular endothelial growth factor signaling pathway[J]. Eur J Dermatol, 2013, 23(2):274-275. http://europepmc.org/abstract/med/23518371 [14] 阮寒光, 史芳, 汪华, 等.甲磺酸阿帕替尼治疗二线化疗失败后晚期胃癌疗效观察[J].广东医学, 2017, 38(19):3019-3022. doi: 10.3969/j.issn.1001-9448.2017.19.028 [15] Li J, Qin S, Xu J, et al. Randomized, double-blind, placebo-controlled phase Ⅲ trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction[J]. J Clin Oncol. 2016, 34(13):1448-1454. doi: 10.1200/JCO.2015.63.5995 [16] Hu X, Cao J, Hu W, et al. Multicenter phaseⅡstudy of apatinib in non-triple-negative metastatic breast cancer[J]. BMC Cancer, 2014, (14):820. http://cn.bing.com/academic/profile?id=b9f516a30851a76d518f253fb1347eba&encoded=0&v=paper_preview&mkt=zh-cn [17] Hu X, Zhang J, Xu B, et al. Multicenter phaseⅡstudy of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancer[J]. Int J Cancer, 2014, 135(8):1961-1969. doi: 10.1002/ijc.28829 [18] Li J, Qin Sk, Xu JM, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer:results from a randomized, placebo-controlled, parallel-arm, phase Ⅱ trial[J]. J Clin Oncol, 2013, 31(26):3219-3225. doi: 10.1200/JCO.2013.48.8585 [19] Li J, Zhao XM, Chen L, et al. Safety and pharmacokinetics of novelselective vascular endothelial growth factor receptor-2 inhibitor YN968D1 in patients with advanced malignancies[J]. BMC Cancer, 2010, (10):529. doi: 10.1186/1471-2407-10-529. -




 下载:
下载: