Efficacy of axitinib plus sintilimab in intermediate-and high-risk advanced renal cell carcinoma
-
摘要:
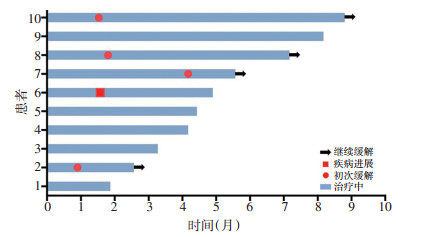
目的 评价阿昔替尼联合信迪利单抗治疗中高危晚期肾癌的初步疗效及安全性。 方法 回顾性分析2019年4月至2019年12月10例就诊于北京大学肿瘤医院行阿昔替尼联合信迪利单抗治疗的晚期肾癌患者的临床资料,其中病理诊断为肾透明细胞癌患者7例、肾非透明细胞癌3例。治疗方案为静脉滴注信迪利单抗200 mg、每3周1次,口服阿昔替尼5 mg、每天2次,并分析客观缓解率、无进展生存期及不良反应。 结果 所有患者中位年龄为58.5(43.0~67.0)岁,国际转移性肾细胞癌数据库联盟(interna?tional metastatic renal cell carcinoma database consortium,IMDC)风险分级均为中危或高危。10例患者的阿昔替尼联合信迪利单抗的客观缓解率为40.0%(4/10),疾病控制率为90.0%(9/10)。7例肾透明细胞癌患者的客观缓解率为57.1%(4/7)。10例患者的主要不良反应中转氨酶升高4例(40.0%),甲状腺功能减退症4例(40.0%),恶心3例(30.0%),高血压2例(20.0%),手足皮肤反应2例(20.0%)。患者的不良反应主要为1~2级,3例发生3~4级不良反应,经对症治疗好转。 结论 阿昔替尼联合信迪利单抗治疗中高危晚期肾癌有较高的客观缓解率,且不良反应多可耐受。 Abstract:Objective To investigate the preliminary efficacy and safety of axitinib plus sintilimab in the treatment of intermediate- and highrisk advanced renal cell carcinoma. Methods A retrospective study of patients with advanced renal cell carcinoma treated with axitinib and sintilimab was conducted in Peking University Cancer Hospital & Institute between April 2019 to December 2019. There were seven cases of clear-cell renal cell carcinoma and three cases of non-clear-cell renal cell carcinoma.All patients received 200 mg sintilimab intravenously every 3 weeks and 5 mg axitinib orally twice daily. Objective response rate (ORR), progression-free survival (PFS), and adverse effects were analyzed. Result With the median age of 58.5 years (range 43.0-67.0), the patients were all classified as intermediate- and high-risk patients, according to the international metastatic renal cell carcinoma database consortium (IMDC) criteria. The overall ORR was 40.0% (4/10), and the disease control rate (DCR) was 90.0% (9/10). The ORR was 57.1% (4/7) in patients with clear-cell renal cell carcinoma. The main adverse effects included elevation of hepatic transaminases (number of patients, n=4; 40.0%), hypothyroidism (n=4; 40.0%), nausea (n=3; 30.0%), hypertension (n=2; 20.0%), and hand-foot skin reaction (n=2; 20.0%). Most adverse effects were of grade 1 or 2, but grade 3-4 adverse events occurred in three patients.Most adverse effects were ameliorated by effective symptomatic treatment. Conclusions Axitinib plus sintilimab achieved promising ORR and DCR in patients with intermediate- and high-risk advanced renal cell carcinoma, with tolerable adverse effects. -
Key words:
- axitinib /
- sintilimab /
- advanced renal cell carcinoma
-
表 1 10例肾癌患者一般特征

表 2 10例肾癌患者行阿昔替尼联合信迪利单抗的不良反应

-
[1] Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6):394-424. doi: 10.3322/caac.21492 [2] Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019[J]. CA Cancer J Clin, 2019, 69(1):7-34. doi: 10.3322/caac.21551 [3] Shi Y, Su H, Song Y, et al. Safety and activity of sintilimab in patients with relapsed or refractory classical Hodgkin lymphoma (ORIENT-1):a multicentre, single-arm, phase 2 trial[J]. Lancet Haematol, 2019, 6(1):e12-e19. doi: 10.1016/S2352-3026(18)30192-3 [4] George S, Rini BI, Hammers HJ. Emerging role of combination immunotherapy in the first- line treatment of advanced renal cell carcinoma:a review[J]. JAMA Oncol, 2019, 5(3):411-421. doi: 10.1001/jamaoncol.2018.4604 [5] Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma[J]. N Engl J Med, 2018, 378(14):1277-1290. doi: 10.1056/NEJMoa1712126 [6] Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma[J]. N Engl J Med, 2019, 380(12):1116-1127. doi: 10.1056/NEJMoa1816714 [7] Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma[J]. N Engl J Med, 2019, 380(12):1103-1115. doi: 10.1056/NEJMoa1816047 [8] Amin A, Plimack ER, Ernstoff MS, et al. Safety and efficacy of nivolumab in combination with sunitinib or pazopanib in advanced or metastatic renal cell carcinoma:the CheckMate 016 study[J]. J Immunother Cancer, 2018, 6(1):109. https://www.ncbi.nlm.nih.gov/pubmed/30348216 [9] Dudek AZ, Liu LC, Gupta S, et al. Phase Ib/II clinical trial of pembrolizumab with bevacizumab for metastatic renal cell carcinoma:BTCRCGU14-003[J]. J Clin Oncol, 2020, 38(11):1138-1145. doi: 10.1200/JCO.19.02394 [10] Taylor MH, Lee CH, Makker V, et al. Phase ⅠB/Ⅱ trial of lenvatinib plus pembrolizumab in patients with advanced renal cell carcinoma, endometrial cancer, and other selected advanced solid tumors[J]. J Clin Oncol, 2020, 38(11):1154-1163. doi: 10.1200/JCO.19.01598 [11] McDermott DF, Huseni MA, Atkins MB, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma[J]. NatMed, 2018, 24(6):749-757. https://www.nature.com/articles/s41591-018-0053-3 [12] Rini BI, Huseni M, Atkins MB, et al. Molecular correlates differentiate response to atezolizumab (atezo) + bevacizumab (bev) vs sunitinib (sun):Results from a phase Ⅲ study (IMmotion151) in untreated metastatic renal cell carcinoma (mRCC)[J]. Ann Oncol, 2018, 29(Suppl 8):viii724-viii725. [13] McGregor BA, McKay RR, Braun DA, et al. Results of a multicenter phase II study of atezolizumab and bevacizumab for patients with metastatic renal cell carcinoma with variant histology and/or sarcomatoid features[J]. J Clin Oncol, 2020, 38(1):63-70. doi: 10.1200/JCO.19.01882 [14] Escudier B, Motzer RJ, Sharma P, et al. Treatment beyond progression in patients with advanced renal cell carcinoma treated with nivolumab in CheckMate 025[J]. Eur Urol, 2017, 72(3):368-376. doi: 10.1016/j.eururo.2017.03.037 [15] Raimondi A, Randon G, Sepe P, et al. The evaluation of response to immunotherapy in metastatic renal cell carcinoma:open challenges in the clinical practice[J]. Int J Mol Sci, 2019, 20(17):4263. doi: 10.3390/ijms20174263 [16] Rini BI, Powles T, Atkins MB, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151):a multicentre, open-label, phase 3, randomised controlled trial[J]. Lancet, 2019, 393(10189):2404-2415. doi: 10.1016/S0140-6736(19)30723-8 -




 下载:
下载: