Management and outcome of hypersensitivity reactions to oxaliplatin: a real-word study in a single center
-
摘要:
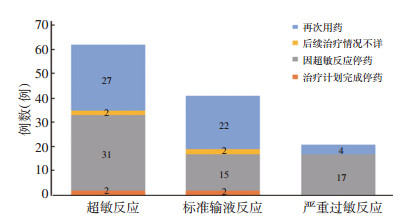
目的 通过对输注奥沙利铂导致的超敏反应的临床特点、处理方式和临床转归进行分析,为临床诊治提供依据。 方法 回顾性分析2015年1月至2017年12月北京大学肿瘤医院上报国家药品不良反应监测中心的62例奥沙利铂超敏反应患者的临床资料,按国际通用标准分型为标准输液反应与严重过敏反应,分析其临床特征、超敏反应亚型、严重程度分级、处理方式及转归。 结果 患者平均年龄(52.9±11.3)岁,男女比例1.07:1。59.7%(37/62)患者在输注奥沙利铂前使用了糖皮质激素预处理。发生超敏反应时奥沙利铂中位治疗为6(4~7.25)个周期,中位累积剂量456.9(263.5~651.0)mg/m2。30.6%(19/62)患者存在无奥沙利铂间隔期。41例(66.1%)患者诊断为标准输液反应,21例(33.9%)诊断为严重过敏反应。在处理方式上,所有患者均采取了暂停用药、更换输液器的处理,无患者使用肾上腺素;所有患者症状均缓解,无死亡病例。58.6%(17/29)需要继续进行治疗的2级标准输液反应患者进行了再次用药,其中70.6%(12/17)未再次出现超敏反应。 结论 临床对奥沙利铂输注前预处理不足,严重过敏反应的肾上腺素的一线使用尚未得到足够重视,大部分轻中度奥沙利铂超敏反应后续可再次安全用药。 Abstract:Objective To review the clinical characteristics of patients with hypersensitivity to oxaliplatin, symptom management, and treatment outcomes to guide further treatment. Methods From January 2015 to December 2017, 62 cases of hypersensitivity reactions to oxaliplatin were reported to the National Center for Adverse Drug Reaction(ADR) Monitoring in the Daycare Center of Peking University Cancer Hospital & Institute. The hypersensitivity reactions were classified into standard infusion-related reactions and anaphylaxis in accordance with international standards. The clinical data, treatment information, and outcomes of these patients were retrospectively collected and analyzed. Results The mean age of the 62 patients was (52.9±11.3) years, the male-to-female radio was 1.07:1, and 59.7% (37/62) of patients received premedication with glucocorticoids before oxaliplatin. The median onset time was 6 (interquartile range 4-7.25) cycles with a median cumulative dose of 456.9 (263.5-651.0) mg/m2. Of the 62 patients, 19 (30.6%) patients had an oxaliplatin-free interval, 41 (66.1%) patients were diagnosed with a standard infusion-related reaction, and 21 (33.9%) patients were diagnosed with anaphylaxis based on clinical criteria. The medication was suspended for all patients and the infusion set was replaced. No patient received epinephrine for symptom management. All patients recovered completely; no deaths were reported. In addition, 58.6% (17/29) of patients with grade 2 standard infusion-related reactions who need further treatment were subsequently rechallenged with oxaliplatin, 70.6% (12/17) showed no symptoms of hypersensitivity. Conclusions Premedication before oxaliplatin was not sufficient and the management of hypersensitivity was not standardized; therefore, the first-line usage of epinephrine should be performed with caution. Most cases of moderate hypersensitivity for soxaliplatin can be rechallenged successfully. -
Key words:
- soxaliplatin /
- hypersensitivity /
- standard infusion-related reaction /
- anaphylaxis /
- rechallenge
-
表 1 不同亚型超敏反应患者的临床特征

表 2 不同亚型超敏反应的临床症状及体征
n(%) 
表 3 不同亚型超敏反应的发生时间、治疗、转归及再用药情况

-
[1] 郑荣寿, 孙可欣, 张思维, 等.2015年中国恶性肿瘤流行情况分析[J].中华肿瘤杂志, 2019, 41(1):19-27. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zhzl201901008 [2] Aroldi F, Prochilo T, Bertocchi P, et al. Oxaliplatin-induced hypersensitivity reaction:underlying mechanisms and management[J]. J Chemother, 2015, 27(2):63-66. https://www.ncbi.nlm.nih.gov/pubmed/25096819 [3] Okayama T, Ishikawa T, Sugatani K, et al. Hypersensitivity reactions to oxaliplatin:identifying the risk factors and judging the efficacy of a desensitization protocol[J]. Clin Ther, 2015, 37(6):1259-1269. https://www.ncbi.nlm.nih.gov/pubmed/25862137 [4] Yamauchi H, Goto T, Takayoshi K, et al. A retrospective analysis of the risk factors for allergic reactions induced by the administration of oxaliplatin[J]. Eur J Cancer Care (Engl), 2015, 24(1):111-116. https://www.ncbi.nlm.nih.gov/pubmed/24304429 [5] Parel M, Ranchon F, Nosbaum A, et al. Hypersensitivity to oxaliplatin:clinical features and risk factors[J]. BMC Pharmacol Toxicol, 2014, 15:1. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3896838/ [6] Shen Y, Li C, Liu W, et al. Clinical analysis of hypersensitivity reactions to oxaliplatin among colorectal cancer patients[J]. Oncol Res, 2018, 26(5):801-807. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=40ddbb0eb460986446e29ce3837dd28f [7] Sohn KH, Kang DY, Kim JY, et al. Incidence and risk of oxaliplatin-induced hypersensitivity in patients with asymptomatic prior exposure:a prospective observational study[J]. J Allergy Clin Immunol Pract, 2018, 6(5):1642-1648. http://www.sciencedirect.com/science/article/pii/S2213219818300114 [8] Bano N, Najam R, Qazi F, et al. Clinical features of oxaliplatin induced hypersensitivity reactions and therapeutic approaches[J]. Asian Pac J Cancer Prev, 2016, 17(4):1637-1641. http://journal.waocp.org/article_32288_db515eb35a50152132d0ba95b754d1b5.pdf [9] Yoshida Y, Hirata K, Matsuoka H, et al. A single-arm phase Ⅱ validation study of preventing oxaliplatin-induced hypersensitivity reactions by dexamethasone:the AVOID trial[J]. Drug Des Devel Ther, 2015, 9:6067-6073. http://europepmc.org/articles/PMC4648596 [10] Sampson HA, Munoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis:summary report——second national institute of allergy and infectious disease/food allergy and anaphylaxis network symposium[J]. J Allergy Clin Immunol, 2006, 117(2):391-397. http://www.sciencedirect.com/science/article/pii/S0196064406000837 [11] 李晓桐, 翟所迪, 王强, 等.严重过敏反应急救指南推荐意见[J].药物不良反应杂志, 2019, 21(2):85-91. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=ywblfyzz201902003 [12] Caiado J, Picard M. Diagnostic tools for hypersensitivity to platinum drugs and taxanes:skin testing, specific IgE, and mast cell/basophil mediators[J]. Curr Allergy Asthma Rep, 2014, 14(8):451. https://www.ncbi.nlm.nih.gov/pubmed/24951237 [13] Kidera Y, Satoh T, Ueda S, et al. High-dose dexamethasone plus antihistamine prevents colorectal cancer patients treated with modified FOLFOX6 from hypersensitivity reactions induced by oxaliplatin[J]. Int J Clin Oncol, 2011, 16(3):244-249. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=5e0dca1d359166d4b1deae88630ddfde [14] 李小倩, 杨全良, 何光照, 等.奥沙利铂过敏反应临床特征的研究[J].中国肿瘤临床, 2018, 45(24):1268-1271. http://html.rhhz.net/ZGZLLC/html/2018-24-7.htm [15] Markman M, Zanotti K, Peterson G, et al. Expanded experience with an intradermal skin test to predict for the presence or absence of carboplatin hypersensitivity[J]. J Clin Oncol, 2003, 21(24):4611-4614. http://www.joplink.net/prev/200803/ref/15-006.html [16] Garufi C, Cristaudo A, Vanni B, et al. Skin testing and hypersensitivity reactions to oxaliplatin[J]. Ann Oncol, 2003, 14(3):497-498. https://paperity.org/p/58273714/skin-testing-and-hypersensitivity-reactions-to-oxaliplatin [17] 刘宇鹏, 高俊峰, 刘丽娟, 等.严重过敏反应诊断和治疗中存在的若干问题探讨[J].医学与哲学, 2012, 33(11B):62-63. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=yxyzx201222029 [18] Muraro A, Roberts G, Worm M, et al. Anaphylaxis:guidelines from the european academy of allergy and clinical immunology[J]. Allergy, 2014, 69(8):1026-1045. doi: 10.1111/all.12437 [19] Simons KJ, Simons FE. Epinephrine and its use in anaphylaxis:current issues[J]. Curr Opin Allergy Clin Immunol, 2010, 10(4):354-361. https://www.researchgate.net/publication/44668998_Epinephrine_and_its_use_in_anaphylaxis_Current_issues [20] Sheikh A, Ten Broek V, Brown SG, et al. H1-antihistamines for the treatment of anaphylaxis:Cochrane systematic review[J]. Allergy, 2007, 62(8):830-837. https://www.ncbi.nlm.nih.gov/pubmed/17620060 [21] Choo KJ, Simons E, Sheikh A, Glucocorticoids for the treatment of anaphylaxis:Cochrane systematic review[J]. Allergy, 2010, 65(10):1205-1211. doi: 10.1111/j.1398-9995.2010.02424.x [22] Sheikh A, Shehata YA, Brown SG, et al. Adrenaline for the treatment of anaphylaxis:cochrane systematic review[J]. Allergy, 2009, 64(2):204-212. https://reference.medscape.com/medline/abstract/19178399 [23] Pumphrey RS. Lessons for management of anaphylaxis from a study of fatal reactions[J]. Clin Exp Allergy, 2000, 30(8):1144-1150. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=10.1046/j.1365-2222.2000.00864.x [24] Simons FE, Ardusso LR, Bilo MB, et al. 2012 update:world allergy organization guidelines for the assessment and management of anaphylaxis[J]. Curr Opin Allergy Clin Immunol, 2012, 12(4):389-399. https://www.research.ed.ac.uk/portal/en/publications/2012-update-world-allergy-organization-guidelines-for-the-assessment-and-management-of-anaphylaxis(78ce5e37-0f9b-4447-b963-c2b8528ca01a)/export.html [25] Thomas RR, Quinn MG, Schuler B, et al. Hypersensitivity and idiosyncratic reactions to oxaliplatin[J]. Cancer, 2003, 97(9):2301-2307. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=10.1002/cncr.11901 -




 下载:
下载: