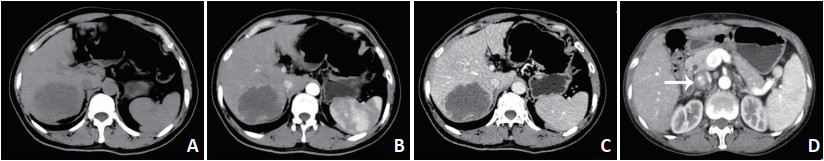
-
摘要:
目的 通过分析各分期肉瘤样肝癌(sarcomatoid hepatocellular carcinoma,SHC)的临床资料、治疗方法及预后,提高临床医生对肉瘤样肝癌的认识。 方法 回顾性分析郑州大学第一附属医院2015年1月至2019年12月病理确诊为SHC 24例患者临床资料并随访,男性16例,女性8例。中位发病年龄55岁。 结果 临床表现以腹痛、发热为主,肝炎病毒感染者占66.7%,肝硬化者占79.2%。Ⅲ~Ⅳ期占87.5%。平均肿瘤长径6.27 cm。1例行肝移植,8例行手术为主治疗,3例仅进行局部治疗,余进行对症支持、化疗、靶向或联合免疫治疗。入院CT或MRI均有异常表现,活检或术后病理均符合SHC。随访1~39.7个月,中位随访时间4.8个月,术后患者中位总生存期(overall survival,OS)为4.7个月,中位无病生存期(disease-free survival,DFS)为2.3个月。Ⅲ~Ⅳ期患者中位OS为4.8个月。 结论 对于Ⅰ期肉瘤样肝癌患者,治疗以外科切除为主,术后给予辅助治疗可使患者受益。对于Ⅲ~Ⅳ期肉瘤样肝癌患者,手术获益局限,可行全身化疗、口服靶向药、局部治疗等减轻肿瘤负荷。但无论分期及治疗措施如何,SHC患者普遍预后差。化疗加靶向药物联合免疫治疗可能成为晚期SHC的治疗新方向。 Abstract:Objective By analyzing the clinical characteristics, treatment options, and prognosis of different stages of sarcomatoid hepatocellular carcinoma (SHC), this study aimed to improve clinicians' understanding of SHC. Methods The clinical data of 24 patients with SHC between January 2015 and December 2019 from First Affiliated Hospital of Zhengzhou University were retrospectively analyzed. There were 16 men and 8 women with a median age of 55 years. Results The clinical symptoms were mainly abdominal pain and fever; 66.7% of the patients had hepatitis virus infection and 79.2% had liver cirrhosis. Patients with stage Ⅲ-Ⅳ carcinoma accounted for 87.5% of the study population. The mean largest tumor diameter was 6.27cm. Patients who were followed up were treated at our hospital, of which one patient underwent liver transplantation, eight underwent surgery, three received locoregional therapy only, and the rest received best support care, chemotherapy, or targeted or combined immunotherapy. Computed tomography or magnetic resonance imaging showed abnormal findings, and biopsy or postoperative pathology was consistent with SHC. The median follow-up period was 4.8(1-39.7) months, and the median overall and disease-free survival of postoperative patients was 4.7 and 2.3 months, respectively. The median overall survival (OS) was 4.8 months for patients with stage Ⅲ-Ⅳ carcinoma. Conclusions For patients with stage I SHC, surgical resection is the preferred treatment. Postoperative adjuvant therapy can benefit patients. For patients with stage Ⅲ-Ⅳ SHC, the benefits of surgery are limited. Systemic chemotherapy, oral targeted drug treatment, or local therapy is feasible for reducing the tumor burden. However, the prognosis of patients with SHC is generally poor, regardless of stage or therapeutic methods. Chemotherapy combined with targeted drugs and immunotherapy may become a new therapeutic direction for advanced SHC. -
表 1 24例SHC患者临床特征

表 2 24例SHC患者的具体资料

-
[1] Liao SH, Su TH, Jeng YM, et al. Clinical manifestations and outcomes of patients with sarcomatoid hepatocellular carcinoma[J]. Hepatology, 2019, 69(1):209-221. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=10.1002/hep.30162 [2] Jernigan PL, Wima K, Hanseman DJ, et al. Natural history and treatment trends in hepatocellular carcinoma subtypes: Insights from a national cancer registry[J]. J Surg Oncol, 2015, 112(8):872-876. doi: 10.1002/jso.24083 [3] Wu L, Tsilimigras DI, Farooq A, et al. Management and outcomes among patients with sarcomatoid hepatocellular carcinoma: A population-based analysis[J]. Cancer, 2019, 125(21):3767-3775. doi: 10.1002/cncr.32396 [4] Koo HR, Park MS, Kim MJ, et al. Radiological and clinical features of sarcomatoid hepatocellular carcinoma in 11 cases[J]. J Comput Assist Tomogr, 2008, 32(5): 745-749. doi: 10.1097/RCT.0b013e3181591ccd [5] Dahm HH. Immunohistochemical evaluation of a sarcomatoid hepatocellular carcinoma with osteoclastlike giant cells[J].Diagn Pathol, 2015, 10:40. doi: 10.1186/s13000-015-0274-4 [6] 周远达, 李强, 李慧锴, 等.肝肉瘤样癌8例临床分析[J].中国肿瘤临床, 2014, 41(20):1297-1300. doi: 10.3969/j.issn.1000-8179.20140887 [7] Kan A, Guo RP. The prognosis of subsequent surgical treatment in patients with sarcomatoid carcinoma in the liver: A retrospective study[J]. Int J Surg, 2018, 55:145-151. doi: 10.1016/j.ijsu.2018.05.736 [8] Shi DL, Ma L, Zhao DW, et al. Imaging and clinical features of primary hepatic sarcomatous carcinoma[J]. Cancer Imaging, 2018, 18(1): 36. doi: 10.1186/s40644-018-0171-7 [9] Schneider G, Massmann A, Fries P, et al. MRI of sarcomatoid carcinoma of the liver[J]. European J Radiology Extra, 2005, 54(2):63-67. doi: 10.1016/j.ejrex.2005.03.008 [10] Seo N, Kim MJ, Rhee H. Hepatic sarcomatoid carcinoma: magnetic resonance imaging evaluation by using the liver imaging reporting and data system[J]. Eur Radiol, 2019, 29(7):3761-3771. doi: 10.1007/s00330-019-06052-8 [11] Maeda T, Adachi E, Kajiyama K, et al. Spindle cell hepatocellular carcinoma. A clinicopathologic and immunohistochemical analysis of 15 cases[J]. Cancer, 1996, 77(1):51-57. http://cn.bing.com/academic/profile?id=9d84737edbed16896656b60cb10a5020&encoded=0&v=paper_preview&mkt=zh-cn [12] Murata M, Miyoshi Y, Iwao K, et al. Combined hepatocellular/cholangiocellular carcinoma with sarcomatoid features: genetic analysis for histogenesis[J]. Hepatol Res, 2001, 21(3):220-227. doi: 10.1016/S1386-6346(01)00100-0 [13] Calderaro J, Couchy G, Imbeaud S, et al. Histological subtypes of hepatocellular carcinoma are related to gene mutations and molecular tumour classification[J]. J Hepatol, 2017, 67(4):727-738. doi: 10.1016/j.jhep.2017.05.014 [14] Li ZY, Wu XL, Bi XY, et al. Clinicopathological features and surgical outcomes of four rare subtypes of primary liver carcinoma[J]. Chin J Cancer Res, 2018, 30(3):364-372. doi: 10.21147/j.issn.1000-9604.2018.03.08 [15] 周坦洋, 孙军辉, 张岳林, 等.肝动脉化学疗法栓塞联合选择性门静脉栓塞在肝癌二期切除术中的应用[J].中华消化杂志, 2014, 34(9): 582-588. doi: 10.3760/cma.j.issn.0254-1432.2014.09.002 [16] Lin CC, Chen CL. Living donor liver transplantation for hepatocellular carcinoma achieves better outcomes[J]. Hepatobiliary Surg Nutr, 2016, 5(5):415-421. doi: 10.21037/hbsn.2016.08.02 [17] 熊伟杰, 张新星, 黄媚娟, 等.32例晚期及术后复发肺肉瘤样癌患者的治疗及生存分析[J].四川大学学报(医学版), 2014, 45(2):320-323. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hxykdxxb201402031 [18] 彭彬, 余涛, 郑红波, 等.纳武单抗治疗一例肺肉瘤样癌达完全缓解[J].中国肿瘤生物治疗杂志, 2019, 26(8):925-927. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgzlswzlzz201908017 [19] Finn RS, Qin SK, Ikeda M, et al. IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma[J]. N Engl J Med, 2020, 382(20):1894-1905. http://www.researchgate.net/publication/327464956_IMbrave150_A_randomized_phase_III_study_of_1L_atezolizumab_plus_bevacizumab_vs_sorafenib_in_locally_advanced_or_metastatic_hepatocellular_carcinoma [20] Qin SK, Ren ZG, Meng ZQ, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial[J]. Lancet Oncol, 2020, 21(4):571-580. doi: 10.1016/S1470-2045(20)30011-5 -




 下载:
下载: