Interpretation of the Clinical Practice Guidelines for Pancreatic Cancer 2019 from the Japan Pancreas Society
-
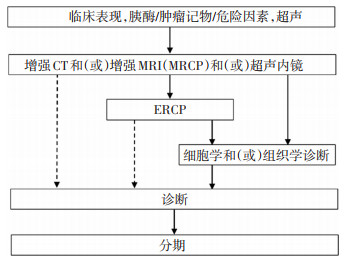
摘要: 日本胰腺协会《胰腺癌临床实践指南》2019年版,为该指南第5次修订更新。日语版于2019年7月发布,2020年3月该指南英文版以大纲模式在“Pancreas”杂志线上发表。指南基于诊断和治疗两大方面,整合为6个板块进行详述,分别为胰腺癌的诊断、可切除性胰腺癌的治疗、交界可切除胰腺癌的治疗、局部进展期胰腺癌的治疗、胰腺癌合并远处转移的治疗、支持与姑息治疗。该指南结合最新文献,在诊断与治疗流程等方面,提出了较为权威的推荐意见,体现出胰腺癌的诊疗现状与研究进展。Abstract: The Clinical Practice Guidelines for Pancreatic Cancer 2019 From the Japan Pancreas Society is the 5th revision of the guidelines. This new revision was published in July 2019, and the English synopsis of these guidelines was published in March 2020. The guidelines are based on the diagnosis and treatment of pancreatic cancer. They address six subjects: diagnosis, treatment of resectable pancreatic cancer, treatment of borderline resectable pancreatic cancer, treatment of locally advanced pancreatic cancer, treatment of locally metastatic pancreatic cancer, and supportive and palliative medicine. The guidelines comprehensively summarized and synthesized the standard clinical practice management of pancreatic cancer and reflected research progress and the current diagnosis and treatment of pancreatic cancer.
-
Key words:
- pancreatic cancer /
- clinical practice guidelines /
- diagnosis /
- treatment
-
[1] Yamaguchi K, Okusaka T, Shimizu K, et al. Clinical practice guidelines for pancreatic cancer 2016 from the japan pancreas society: a synopsis[J]. Pancreas, 2017, 46(5): 595-604. doi: 10.1097/MPA.0000000000000816 [2] Okusaka T, Nakamura M, Yoshida M, et al. Clinical practice guidelines for pancreatic cancer 2019 from the japan pancreas society: a synopsis[J]. Pancreas, 2020, 49(3): 326-335. doi: 10.1097/MPA.0000000000001513 [3] Stoop TF, Ateeb Z, Ghorbani P, et al. Impact of endocrine and exocrine insufficiency on quality of life after total pancreatectomy[J]. Ann Surg Oncol, 2020, 27(2): 587-596. doi: 10.1245/s10434-019-07853-3 [4] Motoi F, Kosuge T, Ueno H, et al. Randomized phase Ⅱ/Ⅲtrial of neoadjuvant chemotherapy with gemcitabine and S- 1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05)[J]. Jpn J Clin Oncol, 2019, 49(2): 190-194. doi: 10.1093/jjco/hyy190 [5] Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open- label, randomised, phase 3 trial[J]. Lancet, 2017, 389 (10073): 1011-1024. doi: 10.1016/S0140-6736(16)32409-6 [6] Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer[J]. N Engl J Med, 2018, 379(25): 2395-2406. doi: 10.1056/NEJMoa1809775 -




 下载:
下载: