Regulation of CX3CR1 expression in pancreatic cancer cells by hypoxia inducible factor 1α
-
摘要:目的 以胰腺癌细胞株Patu8988为研究对象, 通过过表达和干扰低氧诱导因子(HIF-1α), 观察CX3CR1表达水平的变化, 并探讨CX3CR1在胰腺癌中的调控机制。方法 分别构建pcDNA3.1-HIF-1α过表达质粒和HIF-1α-siRNA, 转染胰腺癌细胞株Patu8988, 经Western blot、半定量PCR检测CX3CR1的表达情况。采用染色质免疫沉淀(chromatin immunoprecipitation, ChIP)、荧光素酶技术探查HIF-1α与CX3CR1启动子区的结合情况。结果 Patu8988转染pcDNA3.1-HIF-1α后CX3CR1的表达增加, 敲除HIF-1α后CX3CR1的表达减少。HIF-1α与CX3CR1启动子区的低氧反应元件直接结合, 并上调CX3CR1启动子的活性(P < 0.01)。结论 HIF-1α调控CX3CR1在胰腺癌细胞中的表达。Abstract:Objective This study aimed to investigate the effect of hypoxia inducible factor 1α(HIF-1α) expression on CX3CR1 and its regulatory mechanism in pancreatic cancer cell line Patu8988.Methods The highly expressed plasmid pcDNA3.1 HIF-1α and siRNA HIF-1α were initially constructed. After the plasmid was separately transfected to the pancreatic cancer cells, CX3CR1 and HIF-1α expressions were assayed by western blot analysis and real-time quantitative reverse transcriptase-polymerase chain reaction. The relationship between HIF-1α and CX3CR1 promoter was determined by chromatin immunoprecipitation and luciferase technology.Results The overexpressed HIF-1α could upregulate the CX3CR1 expression in pancreatic cancer cells. The CX3CR1 expression was significantly reduced when HIF-1 α was knocked down. Chromatin immunoprecipitation assay demonstrated that HIF-1α could be directly bound to the hypoxia-response element(5'-A/GCGTG-3') of the CX3CR1 promoter. This binding activity was significantly enhanced under hypoxic condition. CX3CR1 promoter-induced HIF-1α overexpression could significantly upregulate the expression of luciferase reporter genes in pancreatic cancer cells(P < 0.01).Conclusion HIF-1α could regulate CX3CR1 expression in pancreatic cancer cells.
-
Keywords:
- hypoxia inducible factor 1α /
- CX3CR1 /
- pancreatic cancer
-
胰腺癌是一种主要来源于胰腺导管细胞的恶性肿瘤, 其恶性程度极高, 早期即可发生局部浸润和转移, 5年生存率 < 5%[1]。尽管目前手术、放化疗以及新辅助治疗取得很大进展, 但是胰腺癌的中位生存期和5年生存率仍未得到较大的改善。近些年我国胰腺癌的发病率也逐渐升高, 神经痛是胰腺癌的常见症状[2], 严重影响了人类的健康。
HIF-1α在胰腺导管腺癌中高表达[3], 同时CX3CR1也在胰腺导管腺癌中有表达[4]。通过对人类CX3CR1基因5'端旁侧区的基因组DNA片段筛选, 发现了8个低氧反应元件(HREs: 5'-A/GCGTG-3'), 这表明CX3CR1可能是HIF-1α的一个潜在的靶点。本研究拟以胰腺癌细胞株Patu8988为研究对象, 明确HIF-1α对胰腺癌细胞表达CX3CR1的影响。
1. 材料与方法
1.1 主要试剂
DMEM培养基购自Hyclone公司; 胎牛血清购自Gibco公司; 各种PCR引物由上海生物工程有限公司提供; Trizol液、脂质体购自Invitrogen公司; M-MLV逆转录试剂盒购自大连宝生物工程有限公司; HIF-1α小干扰RNA(siHIF1α)由广州锐博生物有限公司设计合成, pcDNA3.1-HIF-1α过表达质粒自备; 抗体HIF-1α购自Santa Cruz(sc-13515)公司, CX3CR1购自Abcam(ab8021)公司, β-actin购自Santa Cruz(sc-47778), CX3CR1-FITC购自Biolegend公司公司; CHIP试剂盒购自Upstate Biotechnology。
胰腺癌细胞系Patu8988由东南大学提供, 37℃、5%CO2饱和湿度条件下培养、传代, 实验用对数生长期细胞, 台盼蓝拒染率在95%以上。转染时, 细胞铺于6孔板, 5×105个/孔, 待细胞长满80%时, 通过脂质体将siHIF-1α或pcDNA3.1-HIF-1α过表达质粒转染到细胞中, 48 h后收集细胞用于后续实验。
1.2 实验方法
Western blot检测蛋白表达: 目的细胞经PBS清洗, 加入含有蛋白酶抑制剂(Sigma)的SDS裂解细胞, 沸水浴10 min, 离心, 经蛋白定量试剂盒(Pierce)定量后, 每孔上样20μg于SDS-PAGE, 以β-actin为内参, 使用相应的抗体检测目的蛋白, ECL法曝光显像。
半定量PCR: TRIzol法抽提细胞RNA。取1μg总RNA, 逆转录得到cDNA。取cDNA 2μL作为模板直接用于普通PCR扩增, 反应体系(25μL)为premix12.5μL, cDNA 2μL, 上下引物各1μL, DEPC水8.5μL。反应程序: 95℃5min; 95℃30 s、55~60℃1 min、72℃1 min, 26个循环; 72℃10 min。PCR物琼脂糖凝胶电泳。图像采集, 结果分析。
ChIP: Patu8988细胞在低氧(1%O2)及常氧(21%O2)条件下的染色体免疫共沉淀, 根据所购试剂盒说明书操作, 以VEGF启动子引物为阳性对照。启动子区PCR引物: CX3CR1:5'-ATTCAGCAGATATAGGGCAGACAGTCAGCTCTCATTAATG-3';VEGF: 5'-GCCTCTGTCTGCCCAGCTGCGTGGAGCTGAGAACGGGAAGC-3'。通过anti-HIF-1α抗体拉下染色体片段, 检测CX3CR1启动子。
荧光素酶实验: 为了验证HIF-1α与CX3CR1启动子的结合是否能够将其激活, 本研究构建了全长的CX3CR1荧光素酶启动子载体, 共转染pGL3-CX3CR1和pcDNA3.1-HIF-1α质粒到Patu8988细胞系中。活性经双荧光素酶报告系统(Promega)测定。
1.3 统计学方法
数据用SPSS17.0统计软件分析, 重复3次独立实验, 数值以mean±SD表示, P < 0.05为差异有统计学意义。
2. 结果
2.1 HIF-1α直接调控CX3CR1的表达
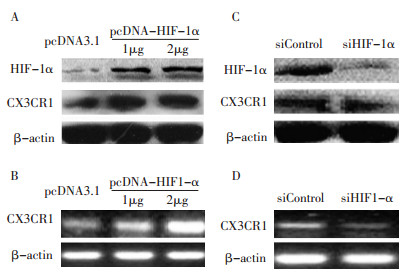
为阐明HIF-1α在CX3CR1表达中的作用, 研究通过转染pcDNA3.1-HIF-1α质粒到胰腺癌细胞系Patu8988上调HIF-1α的表达, 48 h后检测。Western blot结果显示CX3CR1的蛋白水平的表达显著增加, 半定量PCR显示CX3CR1 mRNA表达水平较对照组明显增高(图 1A, B)。
![]() 图 1 过表达和干扰HIF-1α前后CX3CR1的表达变化Figure 1. Changes in CX3CR1 before and after pcDNA3.1 HIF-1α or siHIF-1α was overexpressed and transfectedA: Patu8988 cells transfected with pcDNA3.1 HIF-1α plasmids (1 μg, 2 μg) and assessed by Western blot analysis after 48 h; B: Patu8988 cells transfected with pcDNA3.1 HIF-1 α plasmids (1 μ g, 2 μ g) and assessed by semi-quantitative PCR after 48 h; C: Patu8988 cells transfected with siHIF-1 α (50 nM) and assessed by Western blot analysis after 48 h; D: Patu8988 cells transfected with siHIF-1α (50 nM) and assessed by semi-quantitative PCR after 48 h
图 1 过表达和干扰HIF-1α前后CX3CR1的表达变化Figure 1. Changes in CX3CR1 before and after pcDNA3.1 HIF-1α or siHIF-1α was overexpressed and transfectedA: Patu8988 cells transfected with pcDNA3.1 HIF-1α plasmids (1 μg, 2 μg) and assessed by Western blot analysis after 48 h; B: Patu8988 cells transfected with pcDNA3.1 HIF-1 α plasmids (1 μ g, 2 μ g) and assessed by semi-quantitative PCR after 48 h; C: Patu8988 cells transfected with siHIF-1 α (50 nM) and assessed by Western blot analysis after 48 h; D: Patu8988 cells transfected with siHIF-1α (50 nM) and assessed by semi-quantitative PCR after 48 h经siHIF-1α转染Patu8988胰腺癌细胞以抑制HIF-1α的表达, 48h后Western blot结果显示CX3CR1表达水平较对照组明显降低, 半定量PCR显示CX3CR1mRNA表达水平较对照组明显减少(图 1C, D)。
2.2 HIF-1α直接作用于CX3CR1启动子, 并上调CX3CR1启动子的活性
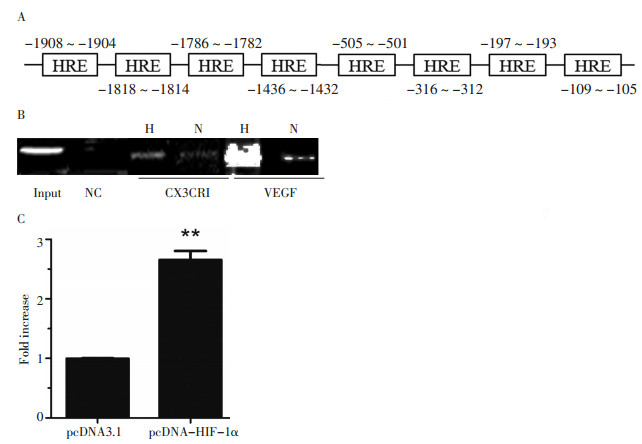
通过对基因组DNA CX3CR1基因5'-旁侧区的筛选, 研究发现了8个潜在的HIF-1结合区(图 2A)。Patu8988细胞系在低氧及常氧条件下的染色体免疫共沉淀证实了细胞中HIF-1α与CX3CR1启动子的结合, 且在低氧条件下二者的结合显著增加(图 2B)。荧光素酶报告分析显示过表达HIF-1α能够显著地增加CX3CR1启动子的活性, 是对照组的2.8倍(P=0.007, 图 2C)。
![]() 图 2 HIF-1α直接结合CX3CR1启动子的HRE区并上调其活性Figure 2. HIF-1αcould directly interact and upregulate CX3CR1 promoter activity▶A: DNA sequence of the CX3CR1 promoter. Hypoxia-response elements were located at different sites; B: Chromatin immunoprecipitation analysis of Patu8988 cells. PCR products of the vascular endothelial growth factor promoter were used as the positive control sample. H, N, and NC indicate hypoxia, normoxia, and negative control, respectively; C: Luciferase analysis of Patu8988 cells. The cells were cotransfected with pGL3-CX3CR1 (1μg) and pcDNA3.1 HIF-1 α plasmids (1μg). Luciferase analysis was performed with a dual-luciferase reporter assay system. Y axis: pGL3-CX3CR1-related luciferase activity. **P < 0.01 versus the control group
图 2 HIF-1α直接结合CX3CR1启动子的HRE区并上调其活性Figure 2. HIF-1αcould directly interact and upregulate CX3CR1 promoter activity▶A: DNA sequence of the CX3CR1 promoter. Hypoxia-response elements were located at different sites; B: Chromatin immunoprecipitation analysis of Patu8988 cells. PCR products of the vascular endothelial growth factor promoter were used as the positive control sample. H, N, and NC indicate hypoxia, normoxia, and negative control, respectively; C: Luciferase analysis of Patu8988 cells. The cells were cotransfected with pGL3-CX3CR1 (1μg) and pcDNA3.1 HIF-1 α plasmids (1μg). Luciferase analysis was performed with a dual-luciferase reporter assay system. Y axis: pGL3-CX3CR1-related luciferase activity. **P < 0.01 versus the control group3. 讨论
CX3CR1在造血细胞[5]、前列腺癌[6]、乳腺癌[7]和胰腺导管腺癌[4]中均有表达。CX3CR1的唯一配体趋化因子CX3CL1[8-9]是一种在神经元和活化的内皮细胞的膜上表达的可溶性的趋化因子[10-11], 在神经元与小胶质细胞的交互中发挥着重要的作用[12]。近期的研究显示, CX3CL1/CX3CR1轴能够调节黏附、迁移, 与肿瘤的神经浸润状态密切相关[13]。
本实验中发现CX3CR1在胰腺癌细胞中高表达。敲除HIF-1α能够减少CX3CR1的表达, 而过表达HIF-1α则能够上调CX3CR1。可见HIF-1α与CX3CR1的表达密切相关。
HIF-1α在许多肿瘤中过度表达, 并与血管生成、细胞代谢、侵袭和转移等密切相关。常氧下胰腺癌细胞表达HIF-1α, 在低氧状态下其表达增高[3]。HIF在调控低氧相关基因中发挥重要作用[14]。作为转录因子, HIF-1α通过激活下游靶基因发挥生物学作用。通过染色体免疫共沉淀和双荧光素酶实验分析, 发现HIF-1α直接与CX3CR1启动子HREs区结合, 上调CX3CR1的转录。进一步证明了HIF-1α可以调控CX3CR1在胰腺癌细胞中的表达。
HIF-1α是否能够通过CX3CR1影响胰腺癌细胞的生物学功能, 将在以后的实验中作进一步的研究证实。此外, 作为胰腺神经浸润的重要细胞因子受体, CX3CR1可由树突细胞、自然杀伤细胞、巨噬细胞、单核细胞产生, 且CX3CR1还与一些免疫相关疾病有关, 那么HIF-1α是否介导了免疫细胞中CX3CR1的表达仍值得进一步研究。
目前已发现多种HIF-1α抑制剂[15], 相比于针对某种特定的趋化因子, 杀死HIF-1α阳性的癌细胞可能会更好地限制胰腺癌的侵袭发展。
-
图 1 过表达和干扰HIF-1α前后CX3CR1的表达变化
Figure 1. Changes in CX3CR1 before and after pcDNA3.1 HIF-1α or siHIF-1α was overexpressed and transfected
A: Patu8988 cells transfected with pcDNA3.1 HIF-1α plasmids (1 μg, 2 μg) and assessed by Western blot analysis after 48 h; B: Patu8988 cells transfected with pcDNA3.1 HIF-1 α plasmids (1 μ g, 2 μ g) and assessed by semi-quantitative PCR after 48 h; C: Patu8988 cells transfected with siHIF-1 α (50 nM) and assessed by Western blot analysis after 48 h; D: Patu8988 cells transfected with siHIF-1α (50 nM) and assessed by semi-quantitative PCR after 48 h
图 2 HIF-1α直接结合CX3CR1启动子的HRE区并上调其活性
Figure 2. HIF-1αcould directly interact and upregulate CX3CR1 promoter activity
▶A: DNA sequence of the CX3CR1 promoter. Hypoxia-response elements were located at different sites; B: Chromatin immunoprecipitation analysis of Patu8988 cells. PCR products of the vascular endothelial growth factor promoter were used as the positive control sample. H, N, and NC indicate hypoxia, normoxia, and negative control, respectively; C: Luciferase analysis of Patu8988 cells. The cells were cotransfected with pGL3-CX3CR1 (1μg) and pcDNA3.1 HIF-1 α plasmids (1μg). Luciferase analysis was performed with a dual-luciferase reporter assay system. Y axis: pGL3-CX3CR1-related luciferase activity. **P < 0.01 versus the control group
-
[1] Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012[J]. CA Cancer J Clin, 2012, 62(1): 10-29. DOI: 10.3322/caac.20138
[2] Gao YJ, Ji RR. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain[J]. Pharmacol Ther, 2010, 126 (1): 56-68. DOI: 10.1016/j.pharmthera.2010.01.002
[3] Zhao T, Gao S, Wang X, et al. Hypoxia-inducible factor-1α regulates chemotactic migration of pancreatic ductal adenocarcinoma cells through directly transactivating the CX3CR1 gene[J]. PLoS One, 2012, 7(8): e43399. DOI: 10.1371/journal.pone.0043399
[4] Marchesi F, Piemonti L, Fedele G, et al. The chemokine receptor CX3CR1 is involved in the neural tropism and malignant behavior of pancreatic ductal adenocarcinoma[J]. Cancer Res, 2008, 68(21): 9060-9069. DOI: 10.1158/0008-5472.CAN-08-1810
[5] Imai T, Hieshima K, Haskell C, et al. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion[J]. Cell, 1997, 91(4): 521-530. DOI: 10.1016/S0092-8674(00)80438-9
[6] Liebig C, Ayala G, Wilks JA, et al. Perineural invasion in cancer: a review of the literature[J]. Cancer, 2009, 115(15): 3379-3391. DOI: 10.1002/cncr.24396
[7] Jamieson-Gladney WL, Zhang Y, Fong AM, et al. The chemokine receptor CX?CR1 is directly involved in the arrest of breast cancer cells to the skeleton[J]. Breast Cancer Res, 2011, 13(5): R91. DOI: 10.1186/bcr3016
[8] Bazan JF, Bacon KB, Hardiman G, et al. A new class of membrane-bound chemokine with a CX3C motif[J]. Nature, 1997, 385 (6617): 640-644. DOI: 10.1038/385640a0
[9] Pan Y, Lloyd C, Zhou H, et al. Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation[J]. Nature, 1997, 387(6633): 611-617. DOI: 10.1038/42491
[10] Haskell CA, Cleary MD, Charo IF. Unique role of the chemokine domain of fractalkine in cell capture. Kinetics of receptor dissociation correlate with cell adhesion[J]. J Biol Chem, 2000, 275(44): 34183-34189. DOI: 10.1074/jbc.M005731200
[11] Marchesi F, Locatelli M, Solinas G, et al. Role of CX3CR1/ CX3CL1 axis in primary and secondary involvement of the nervous system by cancer[J]. J Neuroimmunol, 2010, 224(1-2): 39-44. DOI: 10.1016/j.jneuroim.2010.05.007
[12] Cardona AE, Pioro EP, Sasse ME, et al. Control of microglial neurotoxicity by the fractalkine receptor[J]. Nat Neurosci, 2006, 9(7): 917-924. DOI: 10.1038/nn1715
[13] Marchesi F, Locatelli M, Solinas G, et al. Role of CX3CR1/ CX3CL1 axis in primary and secondary involvement of the nervous system by cancer[J]. J Neuroimmunol, 2010, 224(1-2): 39-44. DOI: 10.1016/j.jneuroim.2010.05.007
[14] Mimura I, Tanaka T, Wada Y, et al. Pathophysiological response to hypoxia - from the molecular mechanisms of malady to drug discovery: epigenetic regulation of the hypoxic response via hypoxia-inducible factor and histone modifying enzymes[J]. J Pharmacol Sci, 2011, 115(4): 453-458. DOI: 10.1254/jphs.10R19FM
[15] Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics[J]. Oncogene, 2010, 29(5): 625-634. DOI: 10.1038/onc.2009.441



 下载:
下载: