Interpretation of the 5th edition (2019) WHO classification of digestive system (appendix and colorectal) tumors
-
摘要: 2019年第5版(以下简称"第5版")世界卫生组织(WHO)消化系统肿瘤新分类在2010年第4版(以下简称"第4版")的基础上对阑尾及结直肠肿瘤的相关内容做了较大的改动,增加了引言、锯齿状病变和息肉、黏液性肿瘤等内容,将不再推荐使用无蒂锯齿状息肉或腺瘤的诊断名称,改称为无蒂锯齿状病变,增加了未分类锯齿状腺瘤。第5版把混合性腺神经内分泌癌改称为混合性神经内分泌-非神经内分泌肿瘤,并指出两者具有克隆相关性,杯状细胞类癌不再被认为是神经内分泌肿瘤的一个亚型,改称为杯状细胞腺癌。第5版进一步细化结直肠腺癌的内容,把血管浸润分为肠壁内血管浸润和肠壁外血管浸润(超出黏膜肌),后者发生率高于前者,而且预后更差。第5版认为肿瘤出芽及差分化癌细胞团是上皮间质转化的征兆,并且应用RNA测序或基于基因芯片技术,以肿瘤基因谱网络和转录组学分析的数据为基础,提出了共识分子分型。同时,第5版也更新了阑尾及结直肠肿瘤的TNM分期。为了帮助医务人员尽快熟悉第5版变更的内容,本文就更新后第4版与第5版的异同进行总结。
-
关键词:
- 消化系统肿瘤 /
- 阑尾 /
- 结肠 /
- 直肠 /
- 世界卫生组织(WHO)分类
Abstract: The contents related to appendix and colorectal tumors in the new 5th edition World Health Organization (WHO) classification of digestive tract tumors in 2019 (hereinafter referred to as "the 5th edition") have considerably changed compared with those in the 4th edition WHO classification in 2010 (hereinafter referred to as "the 4th edition"). In addition, contents related to disease evolution in introduction, serrated lesions, polyps and mucinous tumors, and so on have been newly added. The use of the diagnostic name of sessile serrated polyps or adenomas is no longer recommended in the 5th edition; alternatively, it has been renamed as sessile serrated lesions, and the new term "unclassified serrated adenomas" has been included. The term "mixed adenoneuroendocrine carcinoma" has been replaced with "mixed neuroendocrine-non-neuroendocrine tumors, " and the clonal correlation between the two terms has been emphasized. The goblet cell carcinoid is no longer considered a subtype of neuroendocrine neoplasms; hence, it is renamed goblet cell adenocarcinoma. The 5th edition has further improved content on colorectal adenocarcinoma and categorizes vascular infiltration into intramural and extramural (beyond the mucosal muscles) vascular infiltration. Extramural vascular infiltration, whose prognosis is worse, occurs more frequently than intramural vascular infiltration. Tumor budding or the presence of poorly differentiated clusters is considered as an omen of epithelial-mesenchymal transition. Four main consensus molecular subtype groups have been proposed based on RNA sequencing or gene chip technology and the data from the Cancer Genome Atlas and transcriptome profiling. The TNM staging for appendix and colorectal tumors has also been updated in the 5th edition. The changes in the contents of the 5th edition have been summarized to help medical staff become familiar with them as soon as possible.-
Keywords:
- digestive system neoplasms /
- appendix /
- colon /
- rectum /
- WHO classification
-
2019年第5版(以下简称“第5版”)世界卫生组织(WHO)消化系统肿瘤新分类[1-2]与2010年第4版(以下简称“第4版”)相比,将小肠肿瘤和壶腹部肿瘤合并为一章(第4章),删掉食管-胃交界肿瘤这一章。为了避免内容的重复性,在各个消化器官肿瘤章节里不再逐个阐述可发生于各个消化器官的造血淋巴组织肿瘤、间叶性肿瘤、其他肿瘤(黏膜恶性黑色素瘤、生殖细胞肿瘤、转移瘤)、遗传综合征相关性肿瘤,分别将这4部分的内容独立成章,由此新增4个章节内容(第11、12、13、14章)。第5版共有14个章节,比第4版多了2章,所涉及的腺癌、未分化癌、神经内分泌肿瘤、神经内分泌癌诊断名称增加了非特指(no otherwise specified,NOS)限定词,本文就第5、6章的阑尾及结直肠肿瘤相关内容的主要变化做如下介绍。
1. 阑尾肿瘤
1.1 总体框架的变化
第4版阑尾肿瘤这章包含WHO分类、TNM分期、腺癌、神经内分泌肿瘤、杂类肿瘤5部分内容(表 1),第5版增加引言内容,丰富了锯齿状病变和息肉、黏液性肿瘤等内容并把它们分割出来独立成节描述。明确了定义,指出其具有推挤性边界、黏液性上皮增殖、细胞外黏液共同组织学特征,包括低级别阑尾黏液性肿瘤(编码:8480/1)和高级别黏液性肿瘤(编码:8480/2),涵盖之前的恶性潜能未定的黏液性肿瘤、黏液性囊腺癌、黏液性囊腺瘤。
表 1 WHO第4版与第5版阑尾肿瘤的框架比较
1.2 黏液性肿瘤
当低级别阑尾黏液性肿瘤局限于阑尾,无细胞性黏液或黏液性上皮伸展进入肌层时,其TNM分期归为Tis,累及浆膜下归为T3、T4a及T4b维持不变[3]。高级别黏液性肿瘤罕见,呈微乳头、筛状及实性排列,细胞异型性大,可见坏死,其TNM分期与阑尾腺癌相同。黏液性肿瘤有时出现锯齿状结构,但通常无锯齿状结构的纤细绒毛,既有锯齿状病变,又有低级别阑尾黏液性肿瘤性病变的存在时,推荐归类为低级别阑尾黏液性肿瘤。高、低级别阑尾黏液性肿瘤的发病机理也有区别,前者多见KRAS、GNAS基因突变,APC、TP53、SMAD4、BRAF基因突变少见;后者APC、TP53、SMAD4相对多见,GNAS基因突变少见[4],说明高级别黏液性肿瘤并非从低级别进展而来。阑尾腺癌需分黏液性和非黏液性,包括黏液腺癌、印戒细胞癌,删掉旧版的腺鳞癌,保留NOS未分化癌。阑尾黏液性肿瘤和腹膜的继发性肿瘤的分级一般是统一的,若是两者不一致,需在病理报告中分别予以注明,主要依据细胞的异型程度、印戒细胞和黏液的多寡等进行分级[5] (表 2),腹膜黏液性肿瘤的分级对预后判断的价值高于阑尾肿瘤,即使印戒细胞在腹膜黏液性病变占比 < 50%,但仍具有预后意义,漂浮的印戒细胞预后意义小于印戒细胞的浸润[6]。
表 2 阑尾及腹膜黏液性肿瘤、腺癌分级的组织学标准
1.3 杯状细胞腺癌
杯状细胞类癌(编码:8243/3)的名称改成杯状细胞腺癌(编码:8243/3),不再被认为是神经内分泌肿瘤的一个亚型,可称之为杯状细胞癌,不推荐使用的术语包括杯状细胞类癌、隐窝细胞癌、微腺性腺癌和腺类癌[7-8]。杯状细胞腺癌主要由“高脚杯样”的黏液分泌细胞、数量不等的内分泌细胞和潘氏细胞组成,研究表明与Wnt信号通路基因(USP9X、NOTCH1、CTNNA1、CTNNB1和TRRAP)、染色质重塑基因(ARID1A、ARID2、KDM6A和KMT2D)的突变有关[9-10]。其更具侵袭性,在第5版里另列单独讨论,基于管状或簇状低级别生长模式的占比,推荐3级分级,若占比>75%,列为1级;介于50%~75%,为2级; < 50%,为3级。无论是什么级别,强调在肿瘤中寻见经典低级别的病变区域,才能诊断杯状细胞腺癌,也可借此用于鉴别印戒细胞癌。嗜铬素和突触素免疫组织化学染色突出内分泌细胞数量的变化,但非诊断所必需。
2. 结肠与直肠肿瘤
2.1 总体框架的变化
第5版在第4版基础上增加引言的内容,之后分为良性上皮肿瘤和前体病变、恶性上皮肿瘤两节进行描述,锯齿状病变和息肉、普通型腺瘤、炎症性肠病相关性异型增生独立成节,第4版结直肠癌独立为一节,第5版结直肠腺癌独立为一节(表 3)。
表 3 WHO第4版与第5版结直肠肿瘤的框架比较
2.2 腺癌
2.2.1 组织病理学
旧版腺癌归类在癌项下,与腺鳞癌、梭形细胞癌、鳞状细胞癌、未分化癌并列,第5版删掉鳞状细胞癌、梭形细胞癌,把腺鳞癌、未分化癌归为腺癌的亚型,同时新增腺瘤样腺癌、伴肉瘤样成分的癌等亚型,删掉筛状粉刺型腺癌亚型,其他如髓样癌、微乳头状癌、锯齿状腺癌、低黏附性癌等亚型仍保留。腺瘤样腺癌以前被称为绒毛状腺癌或浸润性乳头状腺癌,≥50%成分为伴绒毛状结构的腺瘤样区,核呈低级别,促纤维间质反应轻微或不易被觉察,推挤性生长,活检标本很难确定是否有浸润癌成分,KRAS基因突变率高,预后好[11]。伴肉瘤样成分的癌成分为未分化或肉瘤样,如梭形细胞、横纹肌样细胞分化的肿瘤,间质常黏液变性,多见SMARCB1缺失表达[12]。未分化癌缺乏推挤性边界、合体细胞生长模式、显著的淋巴浆细胞浸润,可借此与髓样癌鉴别。第5版把血管浸润分为肠壁内血管浸润(intramural vascular invasion,IMVI)和肠壁外血管浸润(超出黏膜肌)(extramural vascular invasion,EMVI),EMVI发生率高于IMVI,预后更差[13]。第5版把肿瘤浸润前沿处出现≤4个的单个、成簇的癌细胞浸润定义为出芽;若≥5个癌细胞浸润且无腺管形成,称作差分化癌细胞团,上述情况的出现提示上皮间质转化的征兆[14],腺癌分级不应该把浸润前沿的成分计入。
2.2.2 发病机制与分子诊断病理学
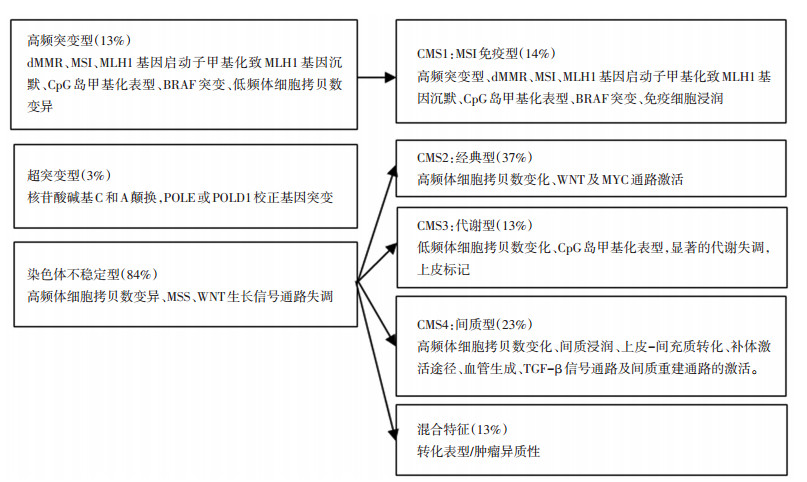
第5版增加了发病机制的内容,丰富了分子病理学的内容,体现近几年在这方面研究所取得的进展。研究表明结直肠癌的发生发展主要涉及染色体不稳定、高频基因突变和超突变这3种途径,其中尤为重要的是应用RNA测序或者基于基因芯片技术,以肿瘤基因谱(TCGA)网络和转录组学分析的数据为基础,提出了共识分子分型(consensus molecular subtype,CMS),其相互之间存在明确的对应关系[15-16](图 1)。
2.2.3 预后和预测
RAS基因、BRAF基因和MSI目前已被研究证实作为指导治疗及其评估预后的生物标记物,PIK3CA、c-Met等为部分得到证实、但尚在研发阶段中的生物标记物[17]。第5版提及有关免疫检查点抑制剂方面的内容,通常高频微卫星不稳定(microsatellite instability-high,MSI-H)肿瘤的肿瘤突变负荷明显增加,是非MSI-H肿瘤的10~100倍,MSHH结直肠癌的肿瘤突变负荷增加尤其显著,因而微卫星不稳定(microsatellite instability,MSI)相比微卫星稳定(microsatellite stability,MSS)结直肠癌患者能够从PD-L1抑制剂免疫治疗中获益[18]。但是目前存在的问题是PD-L1表达的评判采用不同的分析方法和解释标准,“一药一标准”的状况不利于指导临床用药抉择。
2.2.4 炎症性肠病相关性异型增生
第5版独立成节描述,其指的是局限于基底膜内明确的肠上皮肿瘤性改变,分为低级别和高级别。持续的炎症被认为与肿瘤的形成有关,其发病机制一是通过产生引起DNA突变的活性氧,加重组织损伤,破坏关键稳态基因的功能;二是作为肿瘤启动因子在溃疡修复再生过程中促进突变型上皮干细胞或祖细胞的增殖,植入在受累肠段黏膜,在TP53、NF-κB、KRAS和APC等基因的调控下,从中参与肿瘤的发生和发展[19]。
3. 阑尾和结直肠的锯齿状病变和息肉
研究表明,并非所有锯齿状肿瘤性病变在外观上都必需是息肉样隆起[20],第5版不再推荐使用无蒂锯齿状息肉或腺瘤,统称为无蒂锯齿状病变。此外某些锯齿状病变经过仔细的临床/内窥镜和病理评估后仍不能准确地归为增生性息肉、无蒂锯齿状病变、无蒂锯齿状病变伴异型增生、传统型锯齿状腺瘤中的一类,由此增加了未分类锯齿状腺瘤这一类,其相互之间的组织学和分子特征各异[21-26](表 4)。第5版把直径>10 mm的所有腺瘤、管状绒毛状、绒毛状腺瘤和(或)伴有高级别异型增生、黏膜内癌归类为进展性腺瘤(advanced adenoma),临床上须对这些患者进行更严格的监测[27]。第5版保留微泡型、杯状细胞型增生性息肉,然而这两者与锯齿状病变不伴异型增生、进展性息肉均无ICD-O编码,第5版把管状腺瘤、绒毛状腺瘤、管状绒毛状腺瘤依据异型增生程度各分为低级别、高级别。
表 4 锯齿状病变的组织学和分子特征
4. 阑尾和结直肠神经内分泌肿瘤
第5版的L细胞、胰高血糖素样肽和PP/PYY生成性神经内分泌瘤的编码由8152/1改为8152/3,而肠嗜铬细胞类癌、5-羟色胺生成性类癌编码未变(编码:8241/3) [28]。删掉了阑尾管状类癌(编码:8245/1),名称改为管状神经内分泌瘤(罕见),由于许多显微镜下才被偶然发现的病灶往往未能被肉眼发觉,第5版建议将阑尾的整个尖部一分为二纵向剖开全部包埋,目前尚无确定因素以预示阑尾神经内分泌肿瘤的生存预后,即使是采取右半结肠切除术治疗这种肿瘤的生存获益价值也尚未明确。混合性腺神经内分泌癌(编码:8244/3)改成混合性神经内分泌-非神经内分泌肿瘤(编码:8154/3) [29],两种成分具有克隆相关性,若是独立的神经内分泌与非神经内分泌肿瘤的碰撞瘤,不能归类为混合性神经内分泌-非神经内分泌肿瘤,若是腺癌混合神经内分泌癌,仍可称之为混合性腺-神经内分泌癌,新辅助治疗后的标本不诊断混合性神经内分泌-非神经内分泌肿瘤,因为不清楚神经内分泌癌是原来存在或重新发生的,两者的预后意义不相同[30]。
5. 阑尾及结直肠肿瘤的TNM分期
5.1 阑尾分化好的神经内分泌肿瘤TNM分期
第5版根据国际抗癌联盟(UICC)第8版TNM分期(2017年)和美国癌症联合委员会(AJCC)癌症分期的内容进行了修改[31],主要基于肿瘤大小和浆膜/阑尾系膜浸润情况进行分期。T0:无原发肿瘤证据;T1:肿瘤最大径≤2 cm;T2:2 cm < 肿瘤最大径≤4 cm;T3:肿瘤最大径>4cm或浆膜下浸润或侵及阑尾系膜;T4:肿瘤侵透脏层腹膜或侵犯其他器官。N0:无局部淋巴结转移;N1:局部淋巴结转移。M1a:仅肝转移;M1b:仅肝外器官转移;M1c:肝及肝外器官转移。
5.2 结直肠分化好的神经内分泌肿瘤TNM分期
T1、T2和T3分期界定不同于阑尾,N、M分期与阑尾相同[32]。T1:肿瘤浸润固有层或黏膜下层或最大径≤2 cm,T1a < 1 cm,1 cm < T1b≤2 cm;T2:肿瘤浸润肌层或最大径>2 cm;T3:肿瘤浸润浆膜下层或非腹膜覆盖的结直肠周围组织;T4:侵透脏层腹膜或侵犯其他器官。
5.3 阑尾及结直肠癌的TNM分期
不同于神经内分泌肿瘤,神经内分泌癌、混合性神经内分泌-非神经内分泌肿瘤和杯状细胞腺癌按腺癌TNM分期。其中N、M分期在第5版根据UICC第8版TNM分期和AJCC癌症分期做了改动。肿瘤沉积特指在原发肿瘤淋巴引流区域内的孤立肿瘤结节,有研究提示其是结直肠癌预后不良的独立指标,预后介于N1b和N2之间[33],因此其被单独归为N1c。此外进一步细化了M分期,把腹膜癌转移(可同时伴有或者没有其他器官的转移)定义为M1c。
6. 结语
在第4版的基础上,阑尾及结直肠肿瘤的第5版WHO分类综合了近10年的探索、发现及进展,细化组织形态学的描述,深化发病机制及分子病理学等内容,修订了某些肿瘤的命名、性质。临床上特别是消化内、外科及病理科亟需尽快掌握上述内容,与时俱进,更新观念,进一步规范多学科讨论的共同语言,有利于更好地开展相关亚专业的诊疗服务。
-
表 1 WHO第4版与第5版阑尾肿瘤的框架比较

表 2 阑尾及腹膜黏液性肿瘤、腺癌分级的组织学标准

表 3 WHO第4版与第5版结直肠肿瘤的框架比较

表 4 锯齿状病变的组织学和分子特征

-
[1] Nagtegaal ID, Klimstra DS, Wshington MK. WHO Classification of tumours of the digestive system[M]. Lyon (France):International Agency for Research on Cancer Press, 2019:136-156.
[2] Nagtegaal ID, Arends MJ, Odze RD, et al. WHO Classification of tumours of the digestive system[M]. Lyon (France):International Agency for Research on Cancer Press, 2019:158-192.
[3] Umetsu SE, Shafizadeh N, Kakar S. Grading and staging mucinous neoplasms of the appendix:a case series and review of the literature[J]. Hum Pathol, 2017, (69):81-89.
[4] Zhu X, Salhab M, Tomaszewicz K, et al. Heterogeneous mutational profile and prognosis conferred by TP53 mutations in appendiceal mucinous neoplasms[J]. Hum Pathol, 2019, (85):260-269.
[5] Gündoğar Ö, Kımıloğlu E, Komut N, et al. Evaluation of appendiceal mucinous neoplasms with a new classification system and literature review[J]. Turk J Gastroenterol, 2018, 29(5):533-542.
[6] Legué LM, Creemers GJ, de Hingh IHJT, et al. Review:pathology and its clinical relevance of mucinous appendiceal neoplasms and pseudomyxoma peritonei[J]. Clin Colorectal Cancer, 2019, 18(1):1-7. DOI: 10.1016/j.clcc.2018.11.007
[7] Yozu M, Johncilla ME, Srivastava A, et al. Histologic and outcome study supports reclassifying appendiceal goblet cell carcinoids as goblet cell adenocarcinomas, and grading and staging similarly to colonic adenocarcinomas[J]. Am J Surg Pathol, 2018, 42(7):898-910. DOI: 10.1097/PAS.0000000000001056
[8] Wen KW, Hale G, Shafizadeh N, et al. Appendiceal goblet cell carcinoid:common errors in staging and clinical interpretation with a proposal for an improved terminology[J]. Hum Pathol, 2017, (65):187-193.
[9] Johncilla M, Stachler M, Misdraji J, et al. Mutational landscape of goblet cell carcinoids and adenocarcinoma ex goblet cell carcinoids of the appendix is distinct from typical carcinoids and colorectal adenocarcinomas[J]. Mod Pathol, 2018, 31(6):989-996. DOI: 10.1038/s41379-018-0003-0
[10] Wen KW, Grenert JP, Joseph NM, et al. Genomic profile of appendiceal goblet cell carcinoid is distinct compared to appendiceal neuroendocrine tumor and conventional adenocarcinoma[J]. Hum Pathol, 2018, (77):166-174.
[11] Gonzalez RS, Cates JM, Washington MK, et al. Adenoma-like adenocarcinoma:a subtype of colorectal carcinoma with good prognosis, deceptive appearance on biopsy and frequent KRAS mutation[J]. Histopathology, 2016, 68(2):183-190. DOI: 10.1111/his.12725
[12] Lee M, Son HJ, Jang JH, et al. Sarcomatoid carcinoma manifesting as recurrent rectal cancer and mimicking radiation-induced extraskeletal osteosarcoma:A case report[J]. Int J Surg Pathol, 2017, 25(8):732-738. DOI: 10.1177/1066896917715911
[13] Leijssen LGJ, Dinaux AM, Amri R, et al. Impact of intramural and extramural vascular invasion on stage Ⅱ-Ⅲ colon cancer outcomes[J]. J Surg Oncol, 2019, 119(6):749-757. DOI: 10.1002/jso.25367
[14] Barresi V, Reggiani Bonetti L, Ieni A, et al. Poorly differentiated clusters:clinical impact in colorectal cancer[J]. Clin Colorectal Cancer, 2017, 16(1):9-15. DOI: 10.1016/j.clcc.2016.06.002
[15] Thanki K, Nicholls ME, Gajjar A, et al. Consensus molecular subtypes of colorectal cancer and their clinical implications[J]. Int Biol Biomed J, 2017, 3(3):105-111.
[16] Menter DG, Davis JS, Broom BM, et al. Back to the colorectal cancer consensus molecular subtype future[J]. Curr Gastroenterol Rep, 2019, 21(2):5. DOI: 10.1007/s11894-019-0674-9
[17] González N, Prieto I, Del Puerto-Nevado L, et al. 2017 update on the relationship between diabetes and colorectal cancer:epidemiology, potential molecular mechanisms and therapeutic implications[J]. Oncotarget, 2017, 8(11):18456-18485.
[18] Mármol I, Sánchez-de-Diego C, Pradilla Dieste A, et al. Colorectal carcinoma:A general overview and future perspectives in colorectal cancer[J]. Int J Mol Sci, 2017, 18(1):197. DOI: 10.3390/ijms18010197
[19] Svrcek M, Borralho Nunes P, Villanacci V, et al. Clinicopathological and molecular specificities of inflammatory bowel disease-related colorectal neoplastic lesions:the role of inflammation[J]. J Crohns Colitis, 2018, 12(12):1486-1498.
[20] Hashimoto T, Tanaka Y, Ogawa R, et al. Superficially serrated adenoma:a proposal for a novel subtype of colorectal serrated lesion[J]. Mod Pathol, 2018, 31(10):1588-1598. DOI: 10.1038/s41379-018-0069-8
[21] Okamoto K, Kitamura S, Kimura T, et al. Clinicopathological characteristics of serrated polyps as precursors to colorectal cancer:Current status and management[J]. J Gastroenterol Hepatol, 2017, 32(2):358-367. DOI: 10.1111/jgh.13482
[22] Bettington M, Walker N, Rosty C, et al. Clinicopathological and molecular features of sessile serrated adenomas with dysplasia or carcinoma[J]. Gut, 2017, 66(1):97-106. DOI: 10.1136/gutjnl-2015-310456
[23] Geramizadeh B, Robertson S. Serrated polyps of colon and rectum:a clinicopathologic review[J]. J Gastrointest Cancer, 2017, 48(4):291-298. DOI: 10.1007/s12029-017-9977-y
[24] De Palma FDE, D'Argenio V, Pol J, Kroemer G, et al. The molecular hallmarks of the serrated pathway in colorectal cancer[J]. Cancers (Basel), 2019, 11(7):1017. DOI: 10.3390/cancers11071017
[25] Crockett SD, Nagtegaal ID. Terminology molecular features epidemiology and management of serrated colorectal neoplasia[J]. Gastroenterology, 2019, 157(4):949-966. DOI: 10.1053/j.gastro.2019.06.041
[26] Murakami T, Akazawa Y, Yatagai N, et al. Molecular characterization of sessile serrated adenoma/polyps with dysplasia/carcinoma based on immunohistochemistry, next-generation sequencing, and microsatellite instability testing:a case series study[J]. Diagn Pathol, 2018, 13(1):88. DOI: 10.1186/s13000-018-0771-3
[27] Dubé C, Yakubu M, McCurdy BR, et al. Risk of advanced adenoma, colorectal cancer and colorectal cancer mortality in people with lowrisk adenomas at baseline colonoscopy:A systematic review and metaanalysis[J]. Am J Gastroenterol, 2017, 112(12):1790-1801. DOI: 10.1038/ajg.2017.360
[28] Moris D, Tsilimigras DI, Vagios S, et al. Neuroendocrine neoplasms of the appendix:A review of the literature[J]. Anticancer Res, 2018, 38(2):601-611.
[29] De Mestier L, Cros J, Neuzillet C, et al. Digestive system mixed neuroendocrine-non-neuroendocrine neoplasms[J]. Neuroendocrinology, 2017, 105(4):412-425. DOI: 10.1159/000475527
[30] De Mestier L, Cros J. Digestive system mixed neuroendocrine-nonneuroendocrine neoplasms (MiNEN)[J]. Ann Endocrinol (Paris), 2019, 80(3):172-173. DOI: 10.1016/j.ando.2019.04.006
[31] Moris D, Tsilimigras DI, Vagios S, et al. Neuroendocrine neoplasms of the appendix:a review of the literature[J]. Anticancer Res, 2018, 38(2):601-611. http://d.old.wanfangdata.com.cn/OAPaper/oai_pubmedcentral.nih.gov_2807510
[32] Capurso G, Gaujoux S, Pescatori LC, et al. The ENETS TNM staging and grading system accurately predict prognosis in patients with rectal NENs[J]. Dig Liver Dis, 2019, 51(12):1725-1730. DOI: 10.1016/j.dld.2019.07.011
[33] Basnet S, Lou QF, Liu N, et al. Tumor deposit is an independent prognostic indicator in patients who underwent radical resection for colorectal cancer[J]. J Cancer, 2018, 9(21):3979-3985. DOI: 10.7150/jca.27475
-
期刊类型引用(4)
1. 吴晓媚. 阑尾杯状细胞腺癌1例. 实用医技杂志. 2024(08): 603-604+612 .  百度学术
百度学术
2. 刘廷,姚萍. B7-H4、miR-145和β-连环素在结直肠癌组织中的表达及相关性研究. 河北医药. 2022(01): 55-58+63 .  百度学术
百度学术
3. 朱健康,玛伊热·图尔荪,王义霞. 普通白光内镜与高清肠镜+i-Scan模式对结直肠息肉诊断效果对比. 中国误诊学杂志. 2021(04): 306-309 .  百度学术
百度学术
4. 王静芳. 血清MMP-11和MMP-26在结直肠癌诊断中的临床意义. 中国肛肠病杂志. 2020(10): 4-6 .  百度学术
百度学术
其他类型引用(1)



 下载:
下载: