Prediction of complete pathological response to neoadjuvant chemotherapy in patients with breast cancer with reduced microcalcification
-
摘要:目的 探讨乳腺癌患者新辅助化疗(neoadjuvant chemotherapy,NAC)后影响微钙化(mcrocalcification,MC)改变的因素及MC减少与肿瘤病理完全反应(pathological complete response,pCR)的相关性。方法 收集2015年1月至2018年12月天津医科大学肿瘤医院215例乳腺癌患者的临床资料,分为范围改变组及数量改变组,评估影响MC改变的因素。根据MC是否减少进行分组,分为MC范围缩小组及MC数量减少组,分析不同分子分型中MC减少与pCR的相关性。采用受试者工作特征曲线(receiver operat-ing characteristic curve,ROC)评价乳腺X线摄影(mammography,MG)检查中MC减少对pCR敏感性、特异性的预测。结果 MC呈弥散分布,范围>2 cm,数量>20个患者更易发生MC减少。MC范围缩小组较非缩小组易发生pCR。MC数量减少组与非减少组比较差异无统计学意义,分子分型不是MC范围缩小及数量减少与pCR的影响因素。MC范围缩小组预测pCR的敏感度为77.78%、特异度为57.45%(P=0.000 1)。结论 乳腺癌患者NAC后MC改变因素为MC范围、数量及分布,MC范围缩小患者的pCR率高,但MC范围缩小对预测pCR准确性较低,暂不推荐MG检查评估NAC后是否达到pCR。Abstract:Objective To investigate causes and factors affecting microcalcification (MC) changes after neoadjuvant chemotherapy (NAC) in patients with breast cancer and to assess the correlation between MC reduction and complete remission rate [pathological complete response (pCR)] of tumors.Methods Clinical data of 215 patients with breast cancer who visited Tianjin Medical University Cancer Hospital from January 1, 2015 to December 31, 2018 were collected. The patients were grouped according to MC range and number of changes, and factors that affected MC changes were evaluated. According to whether MC decreased or not, the patient group was divided into the MC range and MC number reduction groups, and the correlation between the decrease in MC and pCR, assessed using different molecular typing methods, was analyzed. The sensitivity and specificity of the receiver operating characteristic curve analysis (ROC) were used to evaluate the accuracy of MC reduction in predicting pCR.Results The patients with a distribution of diffuse, initial MC range of >2 cm and MC quantity of >20 were more likely to have an MC reduction. The pCR rate was higher in the group with reduced and non-reduced MC. No significant difference was found between the group with decreased MC and the control group. The decreases in MC according to the different molecular typing methods were independent of factors affecting pCR. Reduction in MC range predicts pCR with a sensitivity of 77.78 and specificity of 57.45 (P=0.0001).Conclusions The change factors of MC in patients with breast cancer after neoadjuvant chemotherapy were the range, number, and distribution of calcification. The pCR rate of the patients with reduced MC was high, but the accuracy of pCR prediction based on reduced MG was low. Thus, mammography was not recommended for evaluating pCR after neoadjuvant chemotherapy.
-
晚期乳腺癌首选治疗方法,主要作用为乳腺癌降期、保乳,获得药物敏感性,指导治疗以改善患者预后。NAC后达到病理完全反应(pathological omplete response,pCR)的患者有显著的生存优势[1-3]。乳腺核磁(magnetic resonance imagine,MRI)因不能准确识别微钙化(mcrocalcification,MC)导致诊断的准确性下降[4]。乳腺X线摄影(mammography,MG)检查有助于提高早期诊断率、降低死亡率,乳腺癌MG检查中最典型的特征是微钙化(mcrocalcification,MC)。NAC影响乳腺癌细胞的代谢,导致MC改变,NAC后MC的形态、分布范围、直径和密度均可发生改变。MC被认为是由细胞坏死引起,NAC后坏死细胞增加会导致NAC的MC反应。研究发现,患者的MC数量减少是NAC的主要反应[5-6]。目前,关于NAC对乳腺癌MC影响的研究较少,MC形成和改变的机制尚不清楚。本研究旨在通过探讨NAC后影响MC改变的因素和条件,分析MC减少与肿瘤病理完全反应(pathological omplete response,pCR)的相关性,评估MC减少对pCR准确性的预测。
1. 材料与方法
1.1 研究对象
收集2015年1月至2018年12月天津医科大学肿瘤医院1 084例行NAC的女性乳腺癌患者的临床病理资料,均行NAC+乳腺癌切除术,MG检查显示MC(包含MC、肿块伴MC、MC伴结构扭曲等)患者行空芯针穿刺活检及术后病理学组织检查,其中869例患者因MG检查结果不准确、缺失被排除,215例纳入分析。根据MG检查中MC变化情况分为范围改变组及数量改变组,分析其临床特征。行NAC后215例患者的MC范围缩少为91例、增加为39例、不变为85例,MC数量减少为99例、增加56例、不变60例。
1.2 方法
1.2.1 MG检查
患者在NAC前后分别进行MG检查,采用标准四视图胶片方法,由放射科和乳腺外科医生评估乳腺MG图像,资深放射科医生阅片时采用相同视图评判对以上结果进行一致评估。根据乳腺影像报告及数据系统(breast imaging-reporting and data system,BI-RADS)对MC的形态、分布、范围、直径和密度等进行分类,并测量范围和计算数量。
1.2.2 乳腺癌分子分型
根据肿瘤的激素受体(hormone receptor,HR)和人表皮生长因子-2(human epidermal growth factor-2,HER-2)情况确定患者的分子分型,HR阳性定义为雌激素受体(estrogen receptor,ER)阳性或孕激素受体(progestrogen receptor,PR)阳性;HR阴性定义为ER阴性和PR阴性。将入组病例分为HR阳性/HER-2阴性、HR阳性/HER-2阳性、HR阴性/HER-2阴性、HR阴性/HER-2阳性4类。
1.2.3 NAC疗效判定
NAC后乳腺癌原发病灶pCR被定义乳腺无浸润性癌成分或者仅残存导管内癌成分。
1.3 统计学分析
采用SPSS 25.0和Med Calc 15.8软件进行统计学分析。多组数据采用单因素分析,采用受试者工作特征曲线(receiver operating characteristic curve,ROC)方法分析乳腺癌MC减少对pCR准确性的预测。P < 0.05为差异具有统计学意义。
2. 结果
2.1 MG检查结果
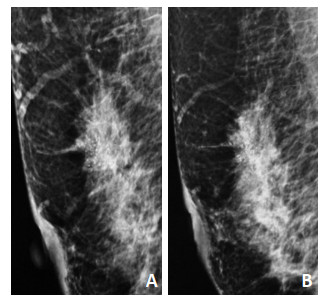
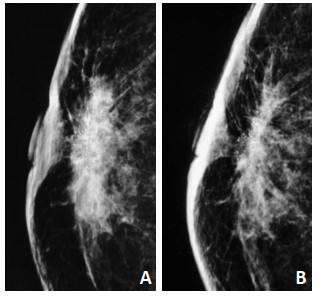
MC范围以最长直径为标准,分为直径 < 2 cm、直径>2 cm,行NAC后MC范围的增大定义为最大直径较前增大≥15%,缩小定义为减少≥15%,不变定义为最大直径增加或减少≤15%(图 1)。MC数量分为≤20个或>20个,以MC清楚形态为1个计数单位,MC数量任意减少为减少,任意增加为增加,其余定义为不变(图 2)。
2.2 影响MC变化的单因素分析
行NAC后,MC的分布、范围、数量与MC变化有关。患者的MC弥散分布、范围>2 cm,数量>20个更易出现MC范围缩小或数量减少,与MC形态学及病理分型相比差异具有统计学意义(表 1)。
表 1 215例乳腺癌患者行NAC后MC的病理特征和MC变化模式
2.3 MC减少与pCR的相关性分析
根据NAC后MC数量减少或范围缩小进行分组发现,215例患者的MC范围缩小组为91例、非缩小组124例,范围缩小患者pCR的发生率高(61.7% vs. 28.3%),两组比较差异具有统计学意义(P=0.022);MC数量减少组为99例、非减少组116例,数量减少组与pCR无显著性相关;分子分型不是MC范围缩小及数量减少与pCR相关性的影响因素(表 2)。
表 2 MC减少与pCR的相关性分析
2.4 MG检查中MC减少对NAC预测的准确性分析
采用ROC方法对MC范围缩小和数量减少与pCR相关性的预测结果显示,MC范围缩小组预测pCR的曲线下面积(area under curve,AUC)值为0.676(95%CI为0.609~0.738),敏感度为77.78%、特异度为57.45%,差异具有统计学意义(P=0.000 1);MC数量减少组的AUC值为0.576(95%CI为0.506~0.642),敏感度为59.26%、特异度为55.85%,差异均具有统计学意义(P=0.000 1)。
3. 讨论
研究表明,行NAC后乳腺癌MC范围和数量主要发生的改变是持续不变、消失甚至增加[7-8]。NAC抑制乳腺癌细胞增殖,降低癌病灶周围的多核巨细胞代谢活性,减少MC灶数量,肿瘤同心性收缩可导致MC范围缩小和密度增加[9-10]。MC改变与多种临床病理因素相关,本研究发现MC的分布状态、范围和数量与MC变化有关。
目前,对于NAC后残留的MC处理尚无共识或指南,行NAC后MG范围缩小和数量减少患者的pCR比例较高。Golan等[11]发现,与MC无改变的患者相比,行NAC后MC数量减少患者的pCR率较高(59% vs. 20%,P < 0.006),但对于是否根据MC的范围缩少,认定肿瘤达到pCR减少手术的切除范围尚未统一。研究发现,MC数量减少与pCR无显著性相关,不能反映NAC有效,MC数量增多亦不能证明无效[12-13]。导致以上结果,是因MC的范围测量及数量计算无统一方法。对于MC研究大多是自定标准,多为范围或数量两个方面[14-15]。本研究通过对MC范围缩小或数量减少与pCR相关性评估发现,MC范围缩小组的pCR率高于非范围缩小组,但以MC范围缩小为标准来预测pCR准确性不高。可能是因尚无对MC数量计算的精确方法,导致根据MC数量减少预测pCR的准确性较低[5]。虽然MC对乳腺癌具有较高的筛查和诊断性能,但在评价和预测NAC的pCR方面作用较差,尚不建议使用MC预测pCR。
综上所述,乳腺癌患者行NAC后MC改变因素为MC范围、数量及分布,MC范围缩小患者的pCR率高,但MC减少对预测pCR准确性较低。本研究因存在部分数据缺失、样本量相对较小、人为测量和评估MC变化的情况,尽管可明显发现NAC引起的改变,但却降低了结果的准确性,并且主观性较强地评估MC改变,未使用图像分析软件,仅分析了MC数量和范围的改变,未评估MC形态及分布状态的改变与pCR的相关性,因此暂不推荐MG检查评估行NAC后是否达到pCR。
-
表 1 215例乳腺癌患者行NAC后MC的病理特征和MC变化模式

表 2 MC减少与pCR的相关性分析

-
[1] Colomer R, Saura C, Sánchez-Rovira P, et al. Neoadjuvant management of early breast cancer:a clinical and investigational position statement[J]. Oncologist, 2019, 24(5):603-611. DOI: 10.1634/theoncologist.2018-0228
[2] 中国乳腺癌新辅助治疗专家组.中国乳腺癌新辅助治疗专家共识(2019年版)[J].中国癌症杂志, 2019, 29(5):390-400. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgazzz201905009 [3] Panato C, Abusamaan K, Bidoli E, et al. Survival after the diagnosis of breast or colorectal cancer in the GAZA strip from 2005 to 2014[J]. BMC Cancer, 2018, 18(1):632. DOI: 10.1186/s12885-018-4552-x
[4] Sener SF, Sargent RE, Lee C, et al. MRI does not predict pathologic complete response after neoadjuvant chemotherapy for breast cancer[J]. J Surg Oncol, 2019, 120(6):903-910. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=0cd7641fae01f6e0b7288acca8997423
[5] Qi X, Chen A, Zhang P, et al. Mammographic calcification can predict outcome in women with breast cancer treated with breast-conserving surgery[J]. Oncol Lett, 2017, 14(1):79-88. DOI: 10.3892/ol.2017.6112
[6] Koning JL, Davenport KP, Poole PS, et al. Breast imaging-reporting and data system (BI-RADS) classification in 51 excised palpable pediatric breast masses[J]. J Pediatr Surg, 2015, 50(10):1746-1750. DOI: 10.1016/j.jpedsurg.2015.02.062
[7] Kim YS, Chang JM, Moon HG, et al. Residual mammographic microcalcifications and enhancing lesions on MRI after neoadjuvant systemic chemotherapy for locally advanced breast cancer:correlation with histopathologic residual tumor size[J]. Ann Surg Oncol, 2016, 23(4):1135-1142. DOI: 10.1245/s10434-015-4993-2
[8] An YY, Kim SH, Kang BJ. Residual microcalcifications after neoadjuvant chemotherapy for locally advanced breast cancer:comparison of the accuracies of mammography and MRI in predicting pathological residual tumor[J]. World J Surg Oncol, 2017, 15(1):198. DOI: 10.1186/s12957-017-1263-8
[9] Gu YL, Pan SM, Ren J, et al. Role of magnetic resonance imaging in detection of pathologic complete remission in breast cancer patients treated with neoadjuvant chemotherapy:a Meta-analysis[J].Clin Breast Cancer, 2017, 17(4):245-255. DOI: 10.1016/j.clbc.2016.12.010
[10] Feliciano Y, Mamtani A, Morrow M, et al. Do calcifications seen on mammography after neoadjuvant chemotherapy for breast cancer always need to be excised[J]?Ann Surg Oncol, 2017, 24(6):1492-1498. DOI: 10.1245/s10434-016-5741-y
[11] Golan O, Amitai Y, Menes T. Does change in microcalcifications with neoadjuvant treatment correlate with pathological tumour response[J]?Clin Radiol, 2016, 71(5):458-463. http://cn.bing.com/academic/profile?id=7ffce276813032158d8c289312372451&encoded=0&v=paper_preview&mkt=zh-cn
[12] Adrada BE, Huo L, Lane DL, et al. Histopathologic correlation of residual mammographic microcalcifications after neoadjuvant chemotherapy for locally advanced breast cancer[J]. Ann Surg Oncol, 2015, 22(4):1111-1117. DOI: 10.1245/s10434-014-4113-8
[13] Li JJ, Chen C, Gu Y, et al. The role of mammographic calcification in the neoadjuvant therapy of breast cancer imaging evaluation[J].PLoS One, 2014, 9(2):e88853. DOI: 10.1371/journal.pone.0088853
[14] Fushimi A, Kudo R, Takeyama H. Do decreased breast microcalcifications after neoadjuvant chemotherapy predict pathologic complete response[J]?Clin Breast Cancer, 2020, 20(1):e82-e88. http://cn.bing.com/academic/profile?id=6b55a96cad1a751953f2d89a083d528b&encoded=0&v=paper_preview&mkt=zh-cn
[15] Yim H, Ha T, Kang DK, et al. Change in microcalcifications on mammography after neoadjuvant chemotherapy in breast cancer patients:correlation with tumor response grade and comparison with lesion extent[J]. Acta Radiol, 2019, 60(2):131-139. DOI: 10.1177/0284185118776491
-
期刊类型引用(4)
1. 林力生,李魁. EC-T新辅助化疗后三阴性乳腺癌病理缓解的影响因素及预后分析. 慢性病学杂志. 2023(02): 242-245+249 .  百度学术
百度学术
2. 马强,杨丽,何建信,张春霞,郝金燕. T1~2期人表皮生长因子受体2阳性型及非阳性型浸润性乳腺癌微钙化特点比较. 临床外科杂志. 2022(02): 156-159 .  百度学术
百度学术
3. 张恒乐,任悦,张晓宇. TP与AP方案在三阴性乳腺癌新辅助化疗中的疗效及预后分析. 中外医学研究. 2022(26): 150-155 .  百度学术
百度学术
4. 冯洁萍,万芸. 乳腺癌新辅助化学治疗后微钙化的变化与治疗反应及病理的相关性分析. 新医学. 2021(07): 530-534 .  百度学术
百度学术
其他类型引用(3)



 下载:
下载: